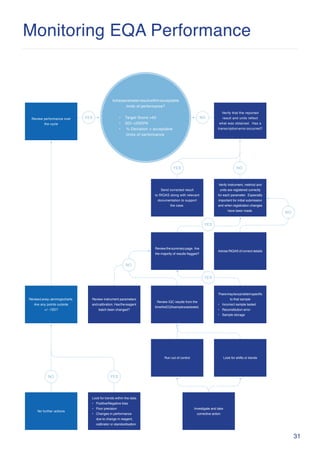
The document outlines a systematic approach for investigating and addressing poor performance in external quality assessment (EQA) programs, highlighting the three main steps: investigating the source of the problem, implementing corrective actions, and checking the effectiveness of those actions. It categorizes analytical errors into clerical, systematic, and random errors, providing guidance on identifying common causes and necessary corrective actions. Additionally, it emphasizes the importance of monitoring performance over time to assess the impact of corrective measures.