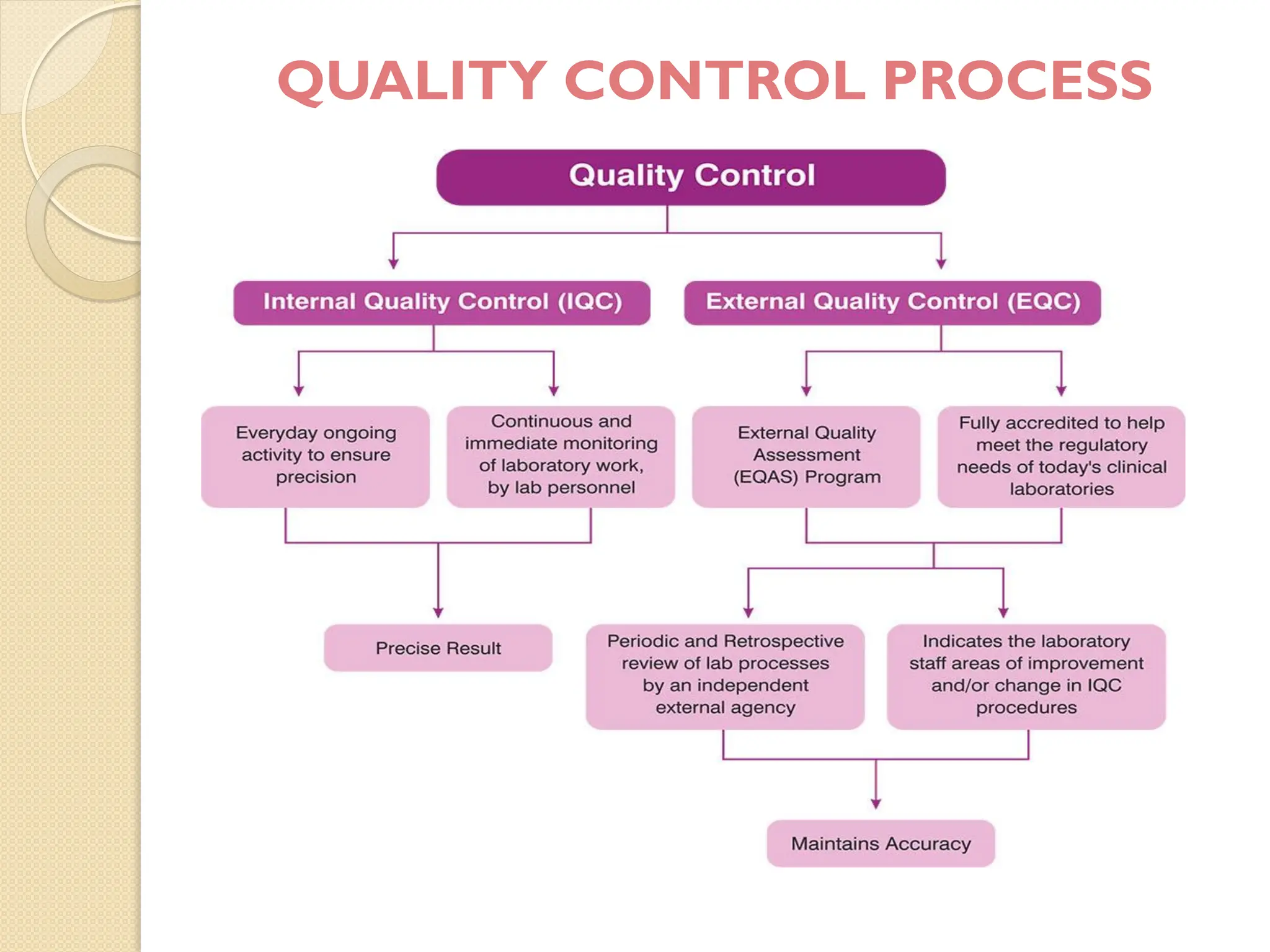
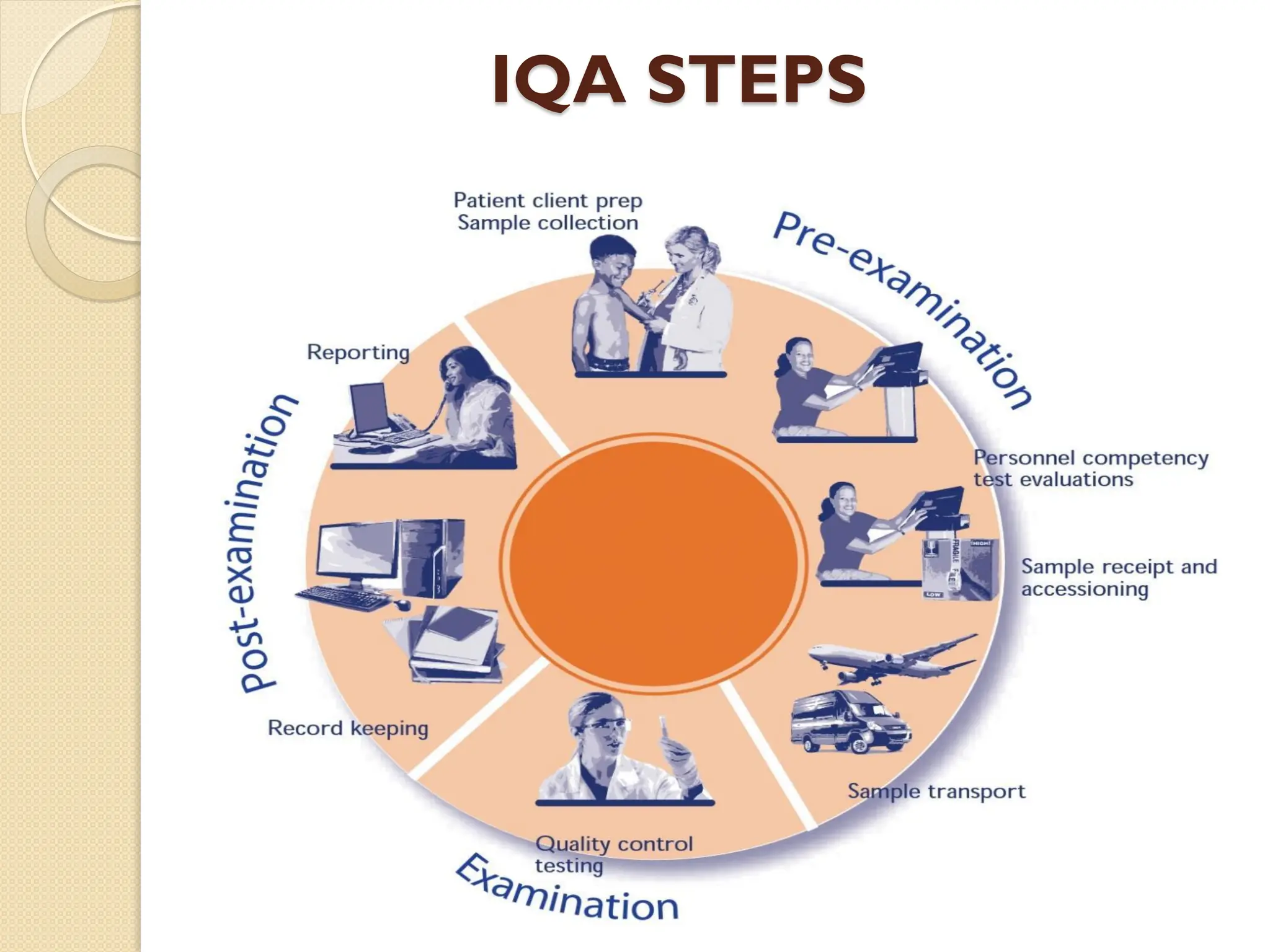
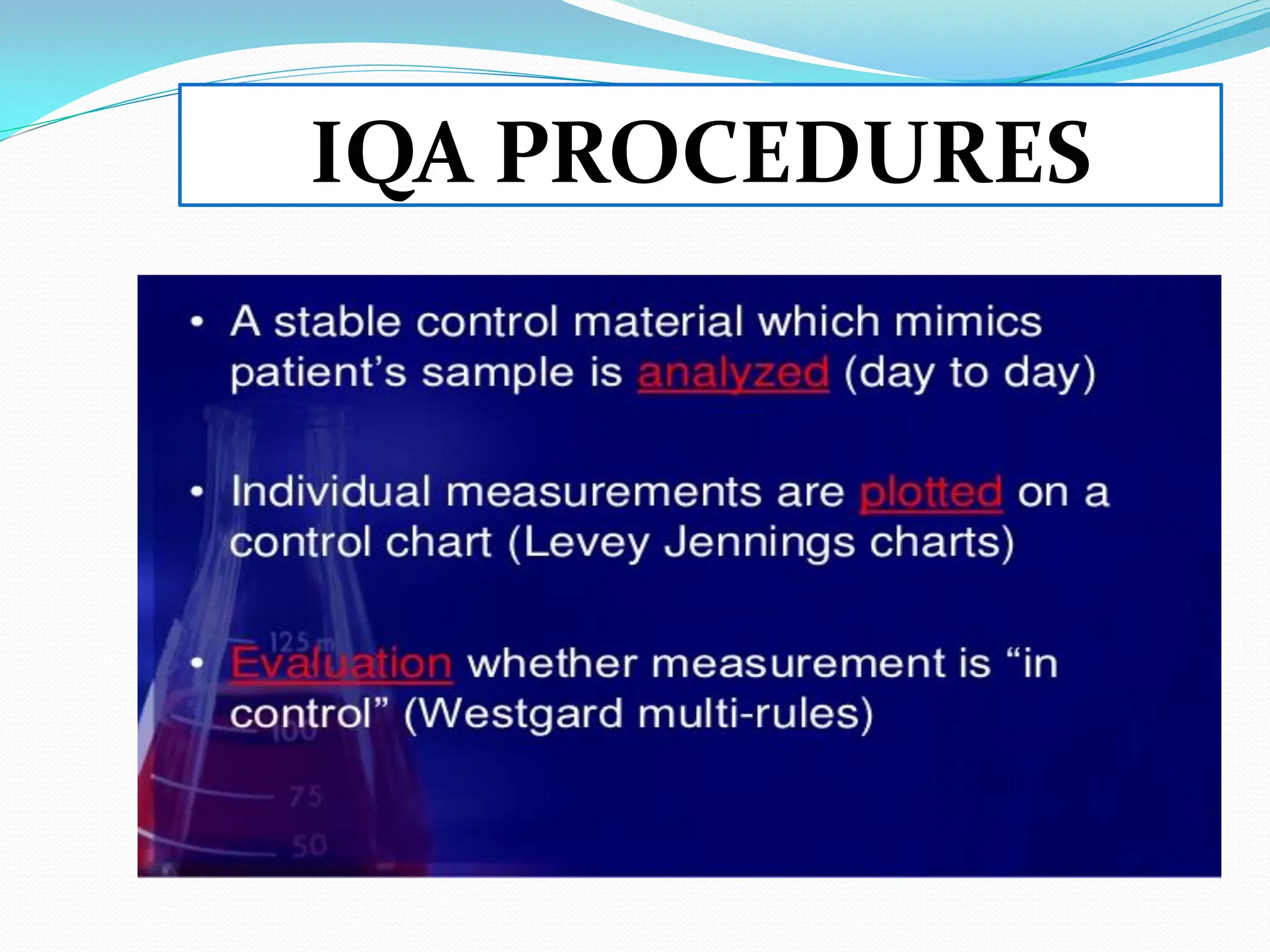
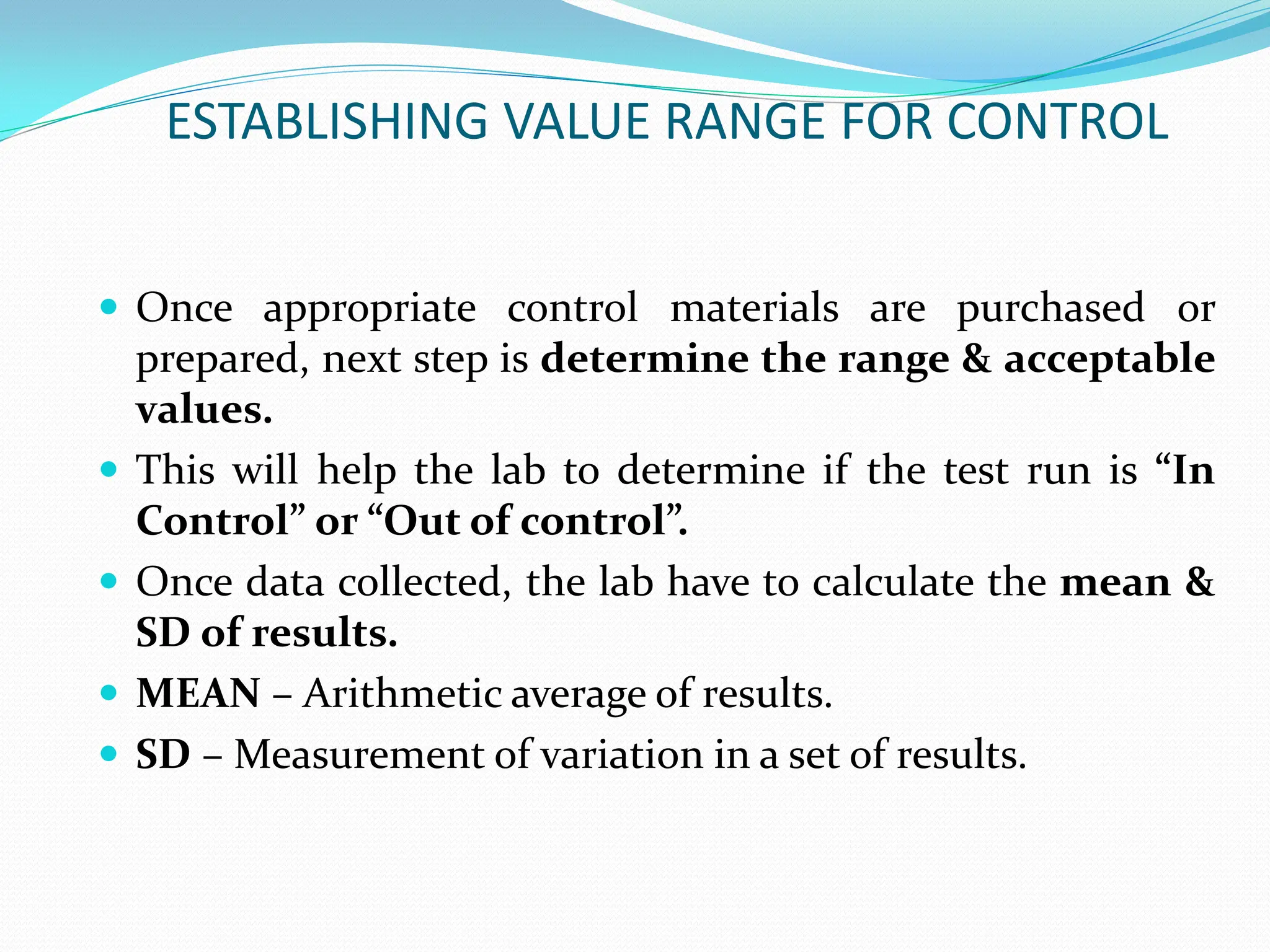
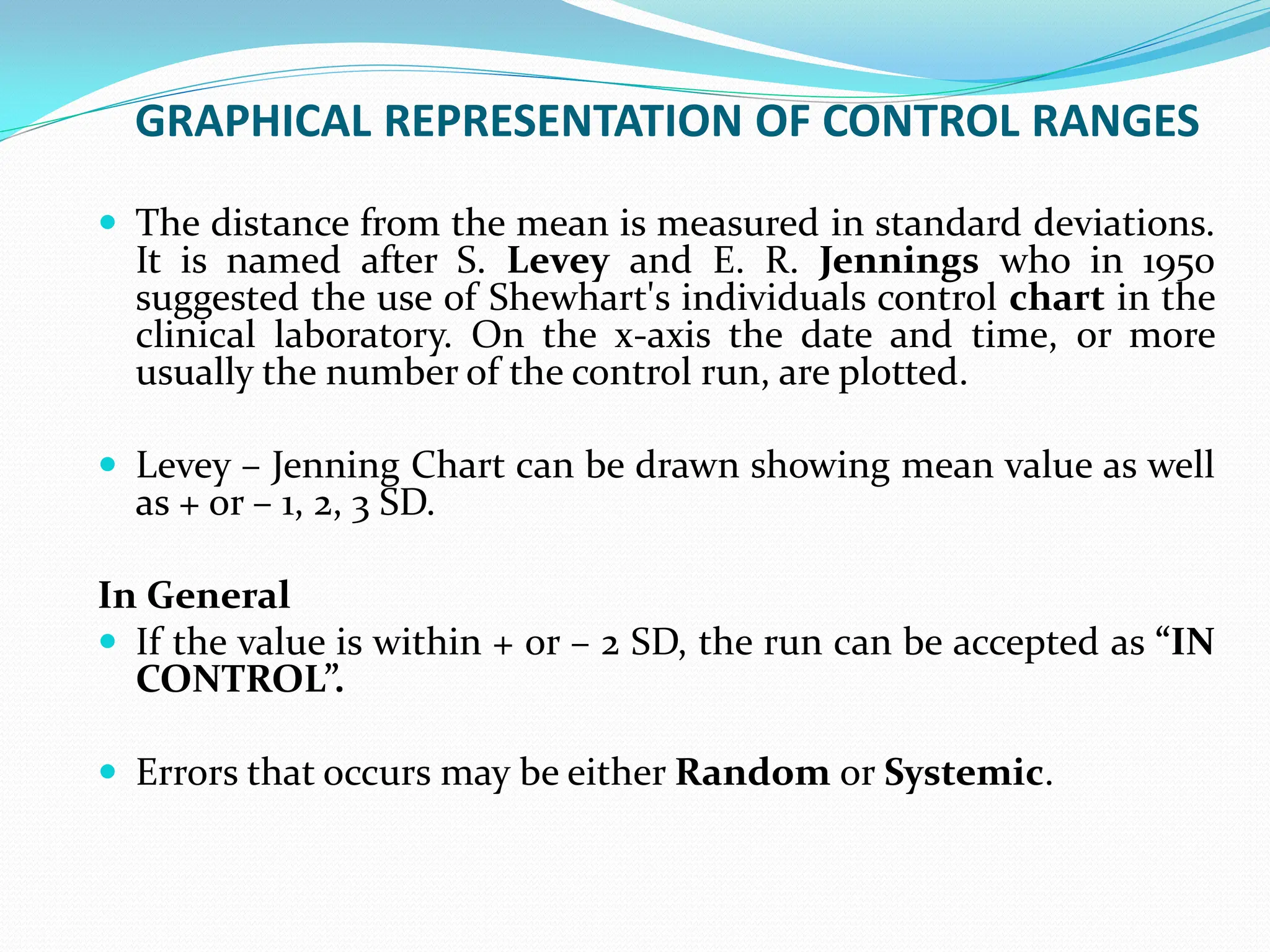
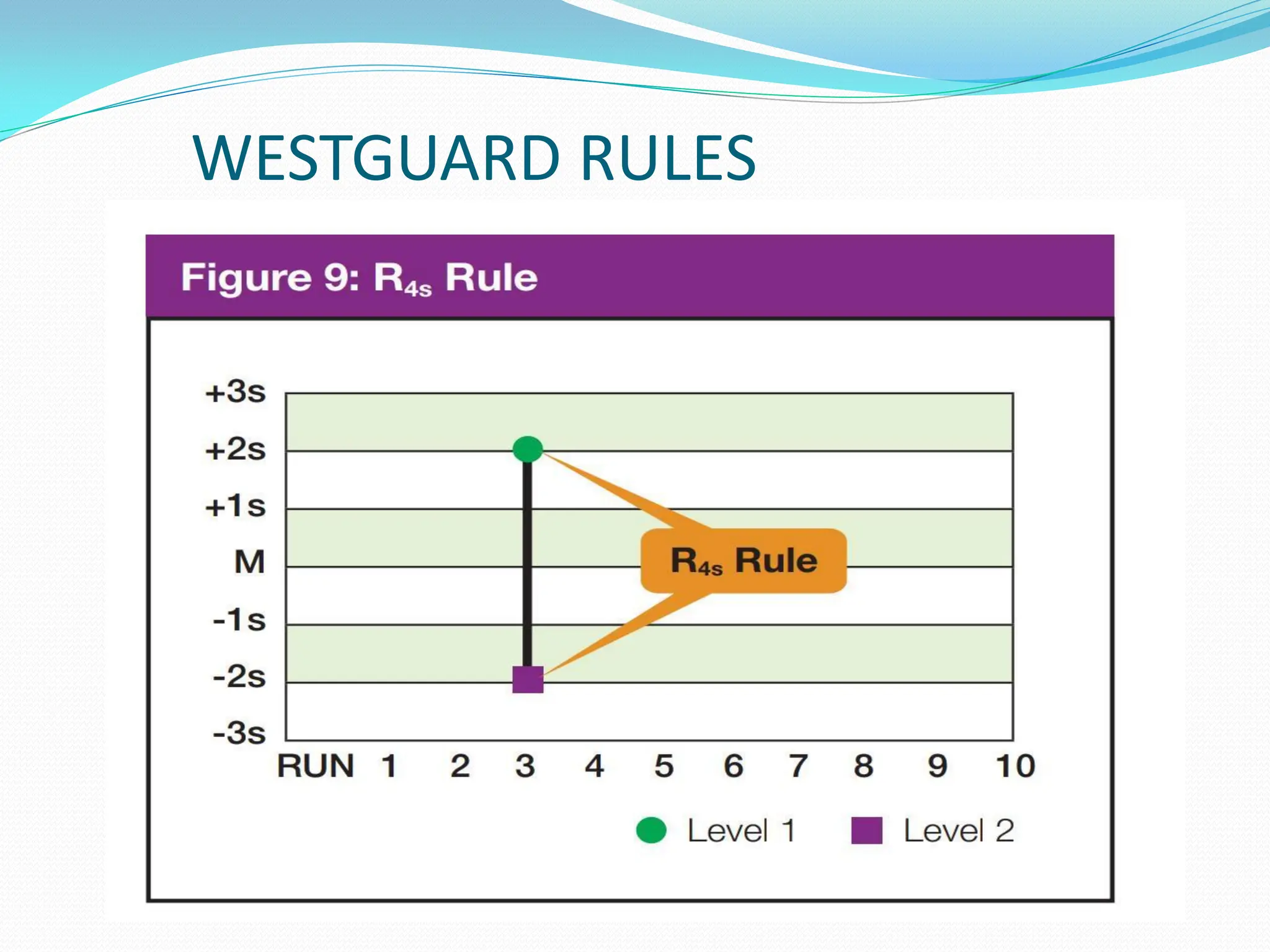
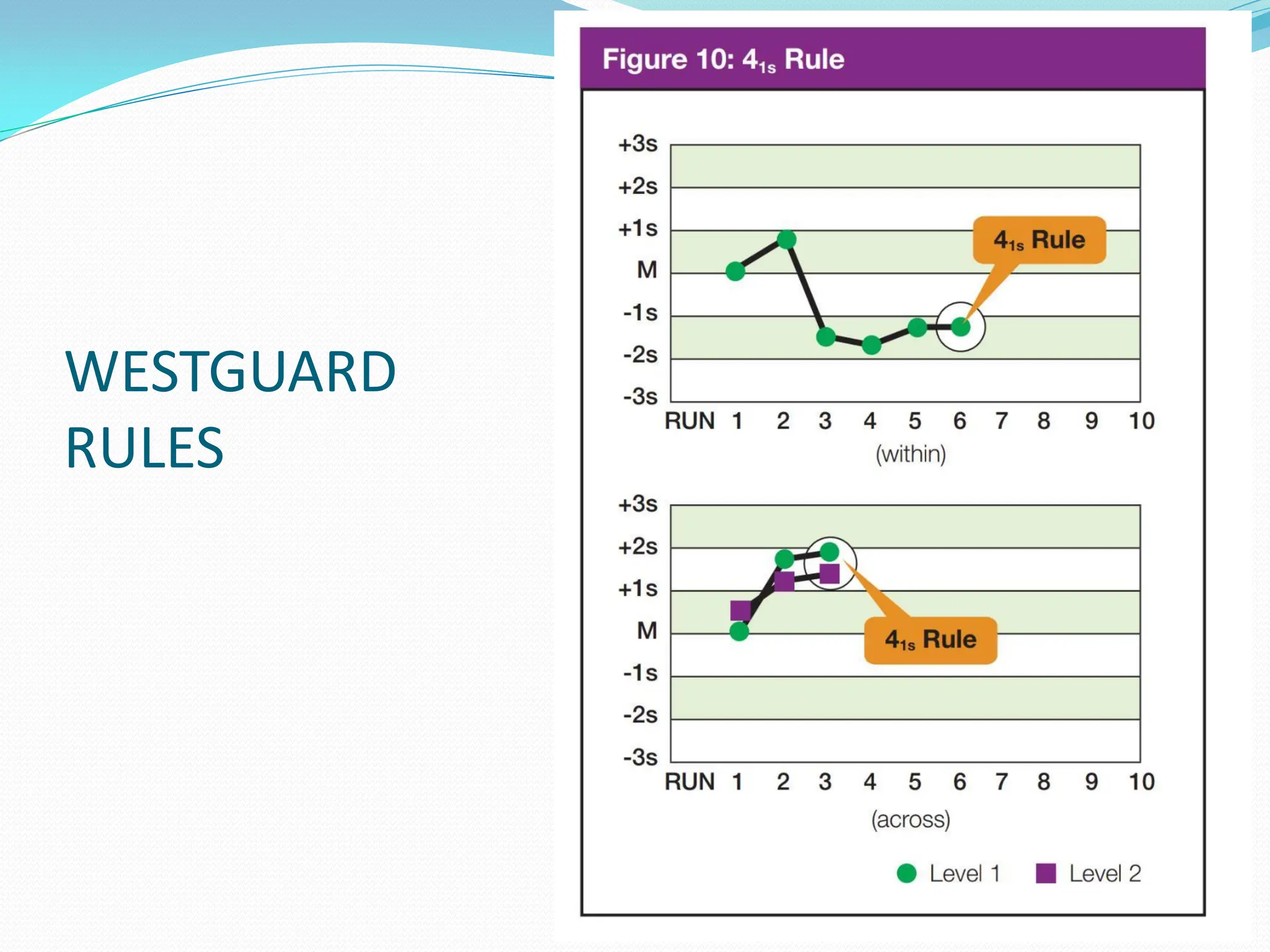
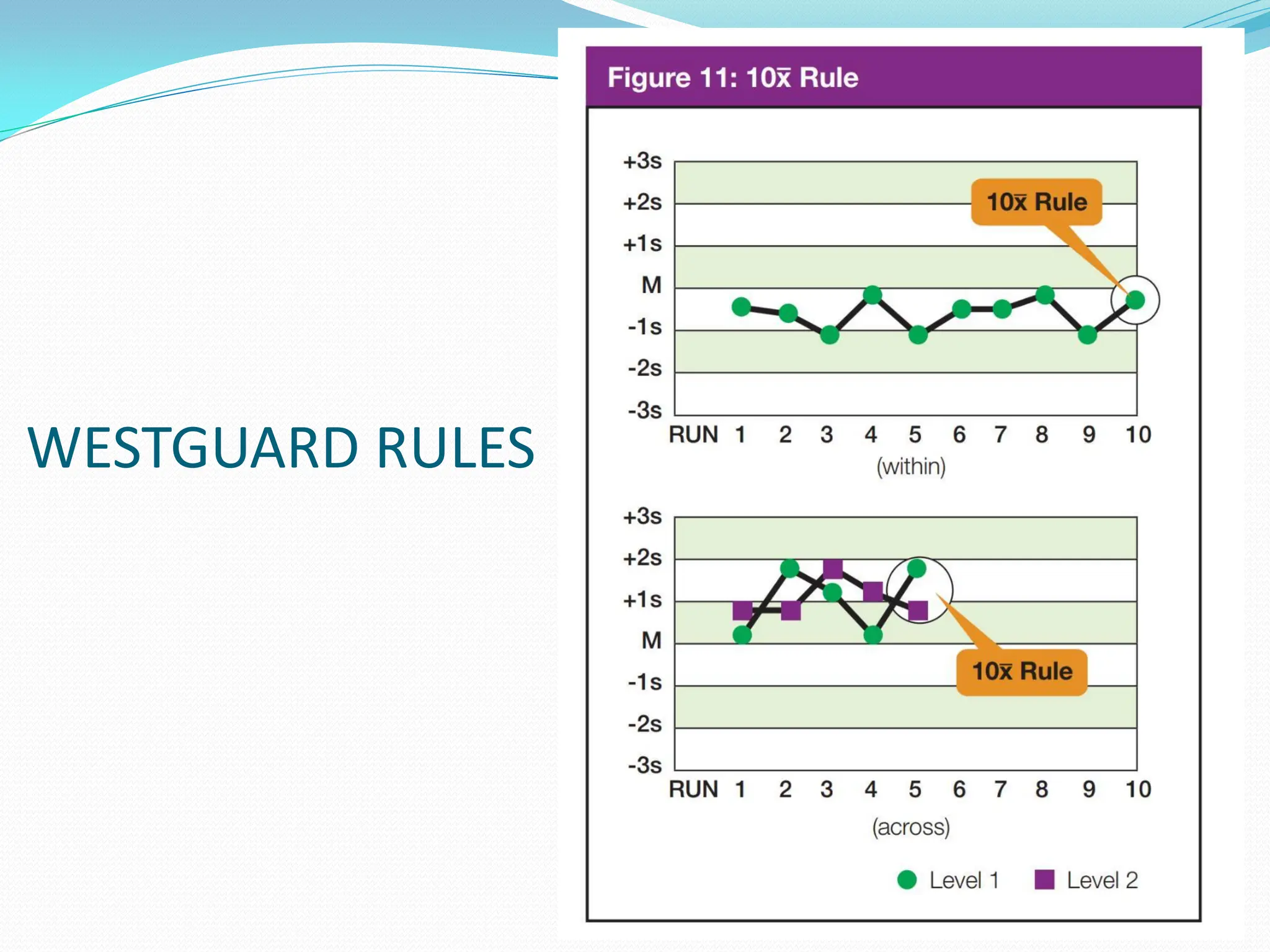
The document outlines a quality assurance (QA) program for medical laboratories, emphasizing procedures to improve the reliability and clinical usefulness of laboratory test results. Key components include internal and external quality controls, training for personnel, and processes to validate testing systems and evaluate operator performance. The ultimate goal is to ensure accurate diagnoses and effective treatments in a clinical setting.