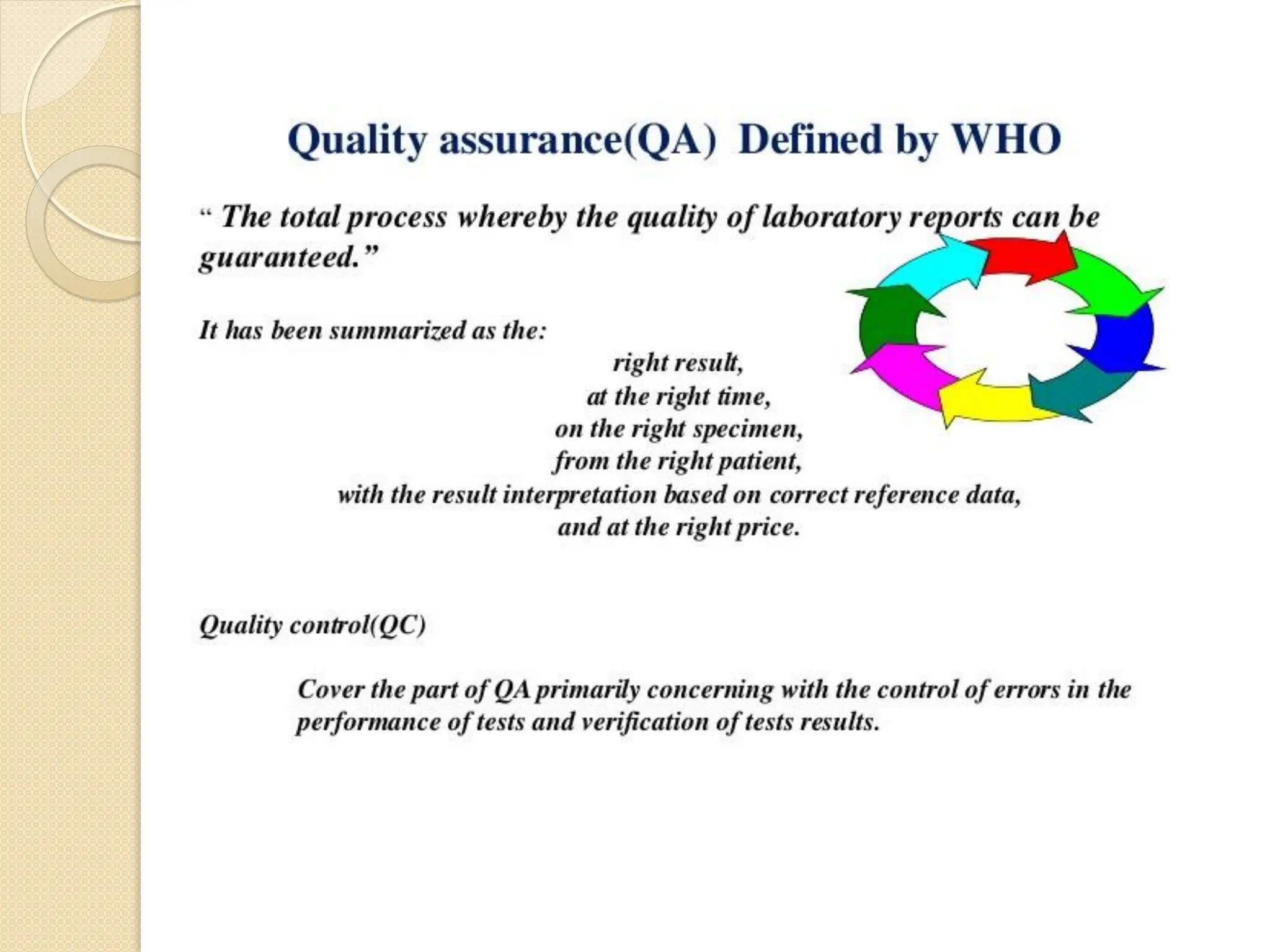
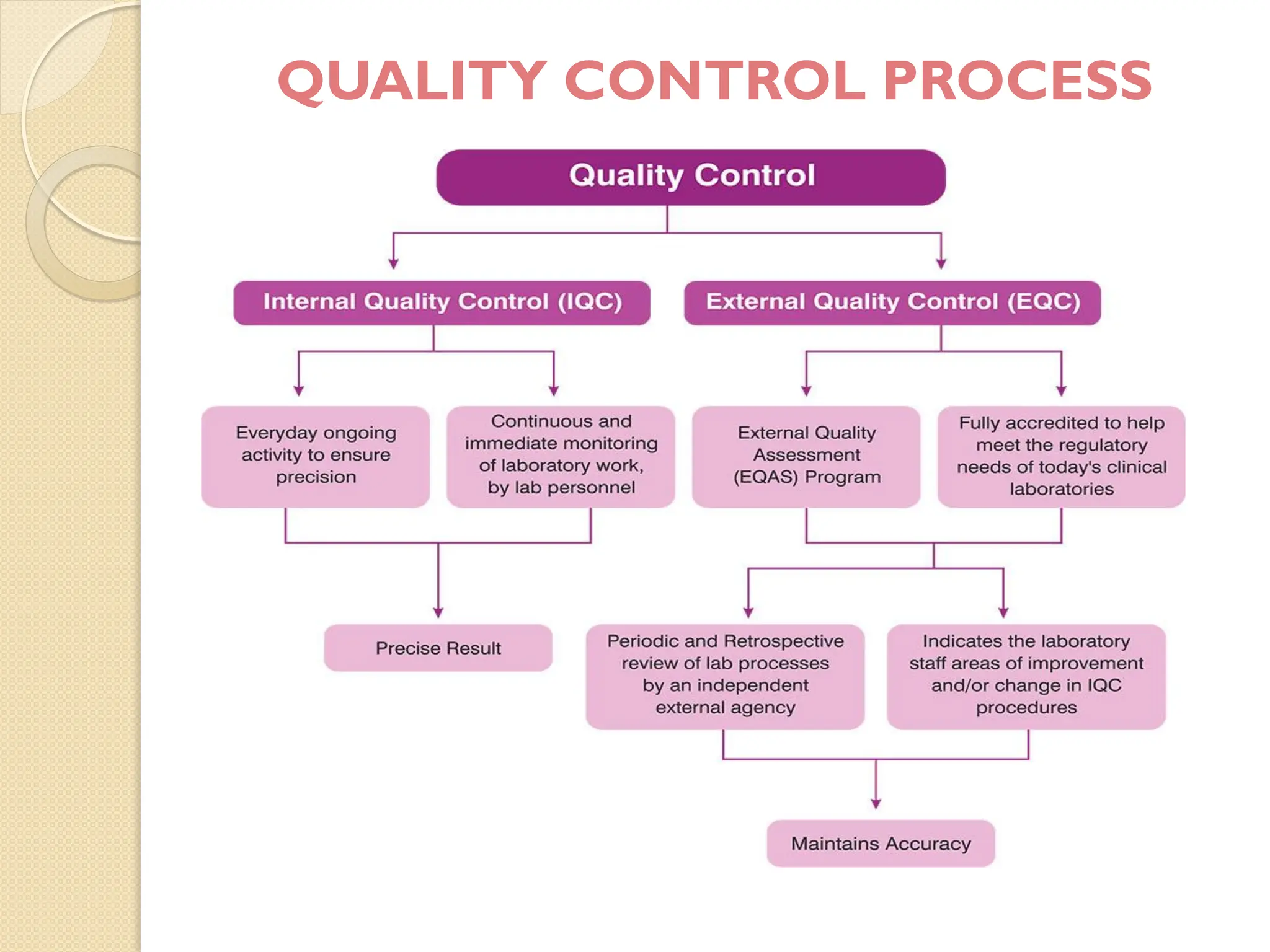
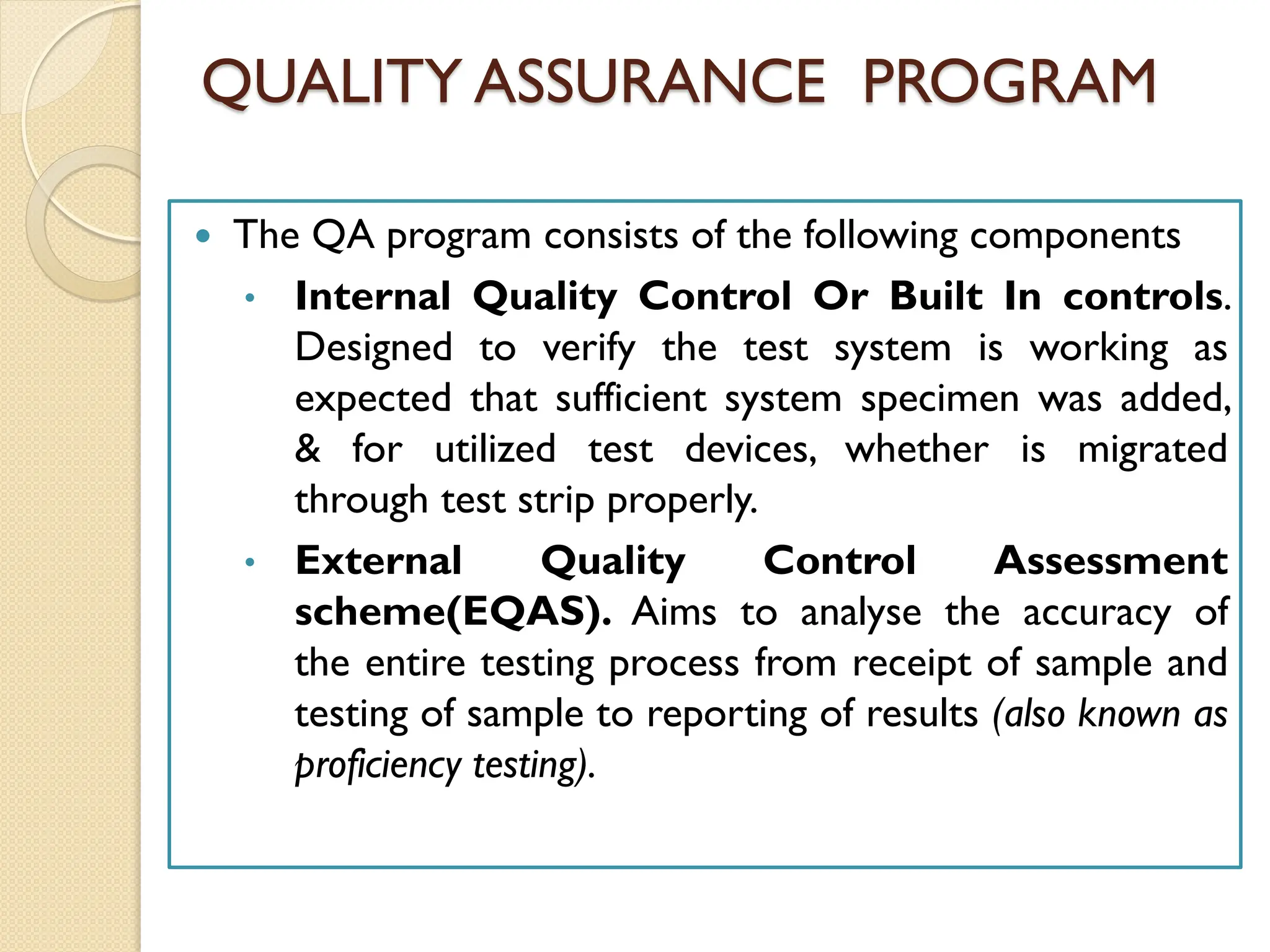
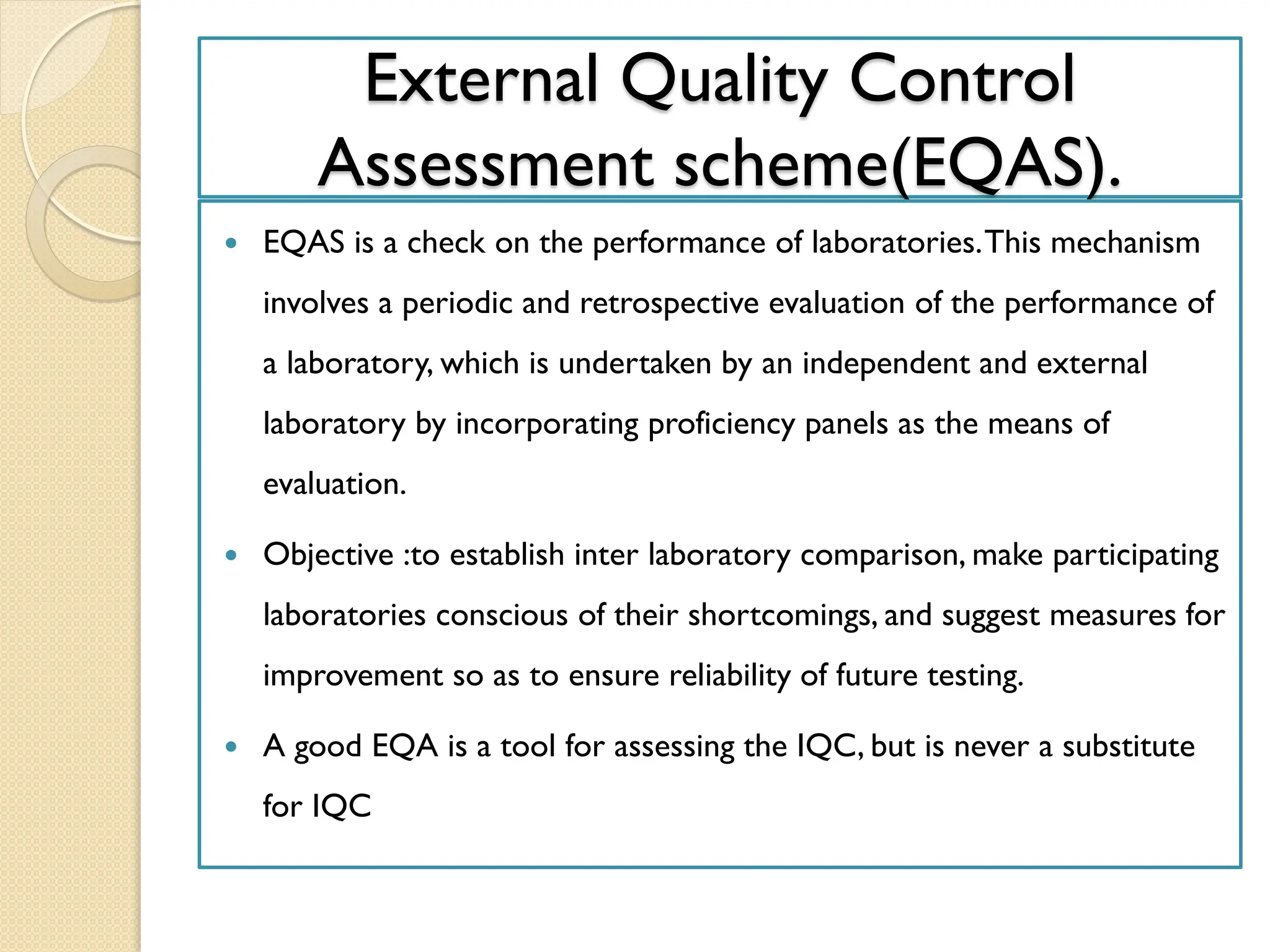
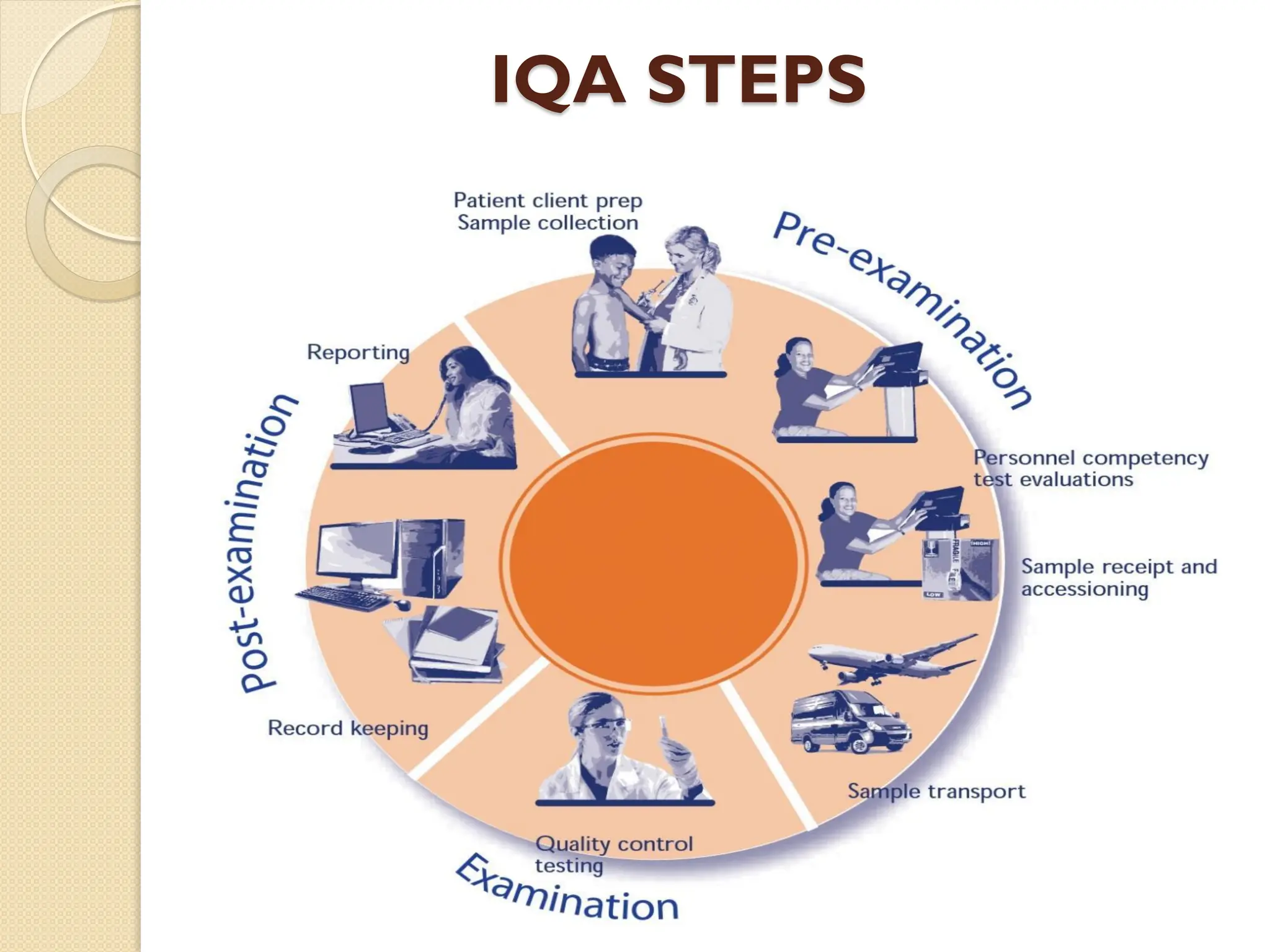
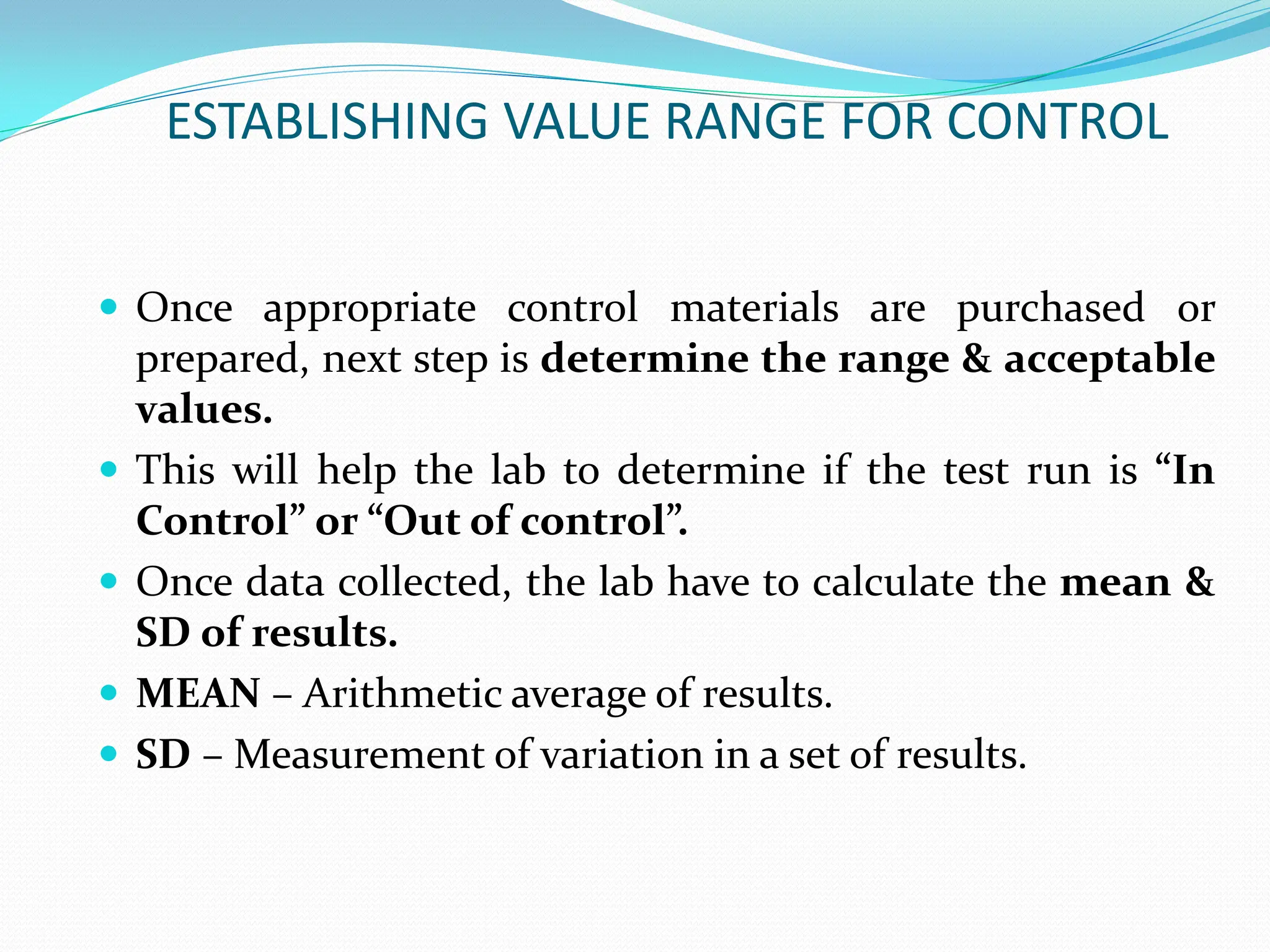
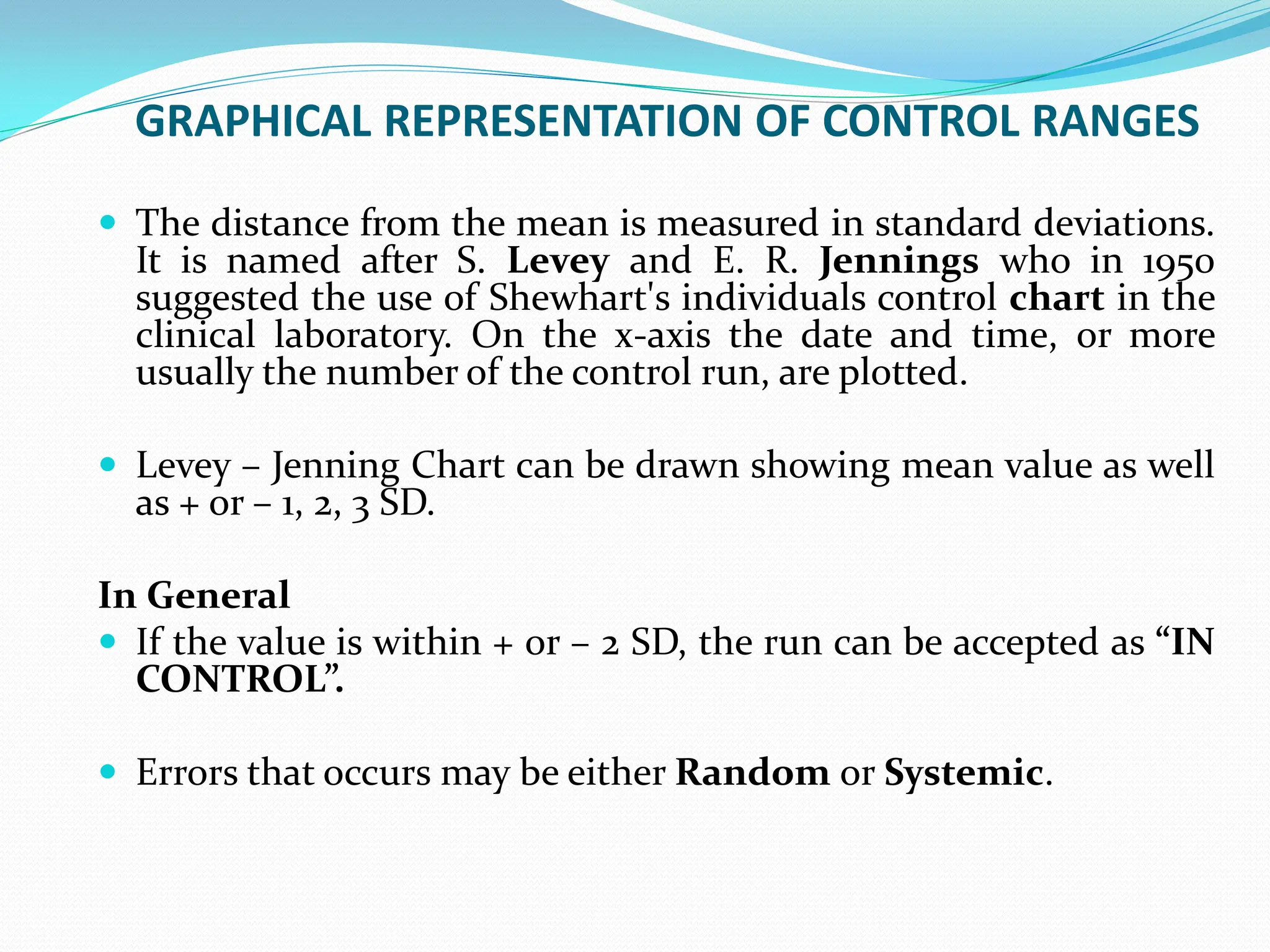
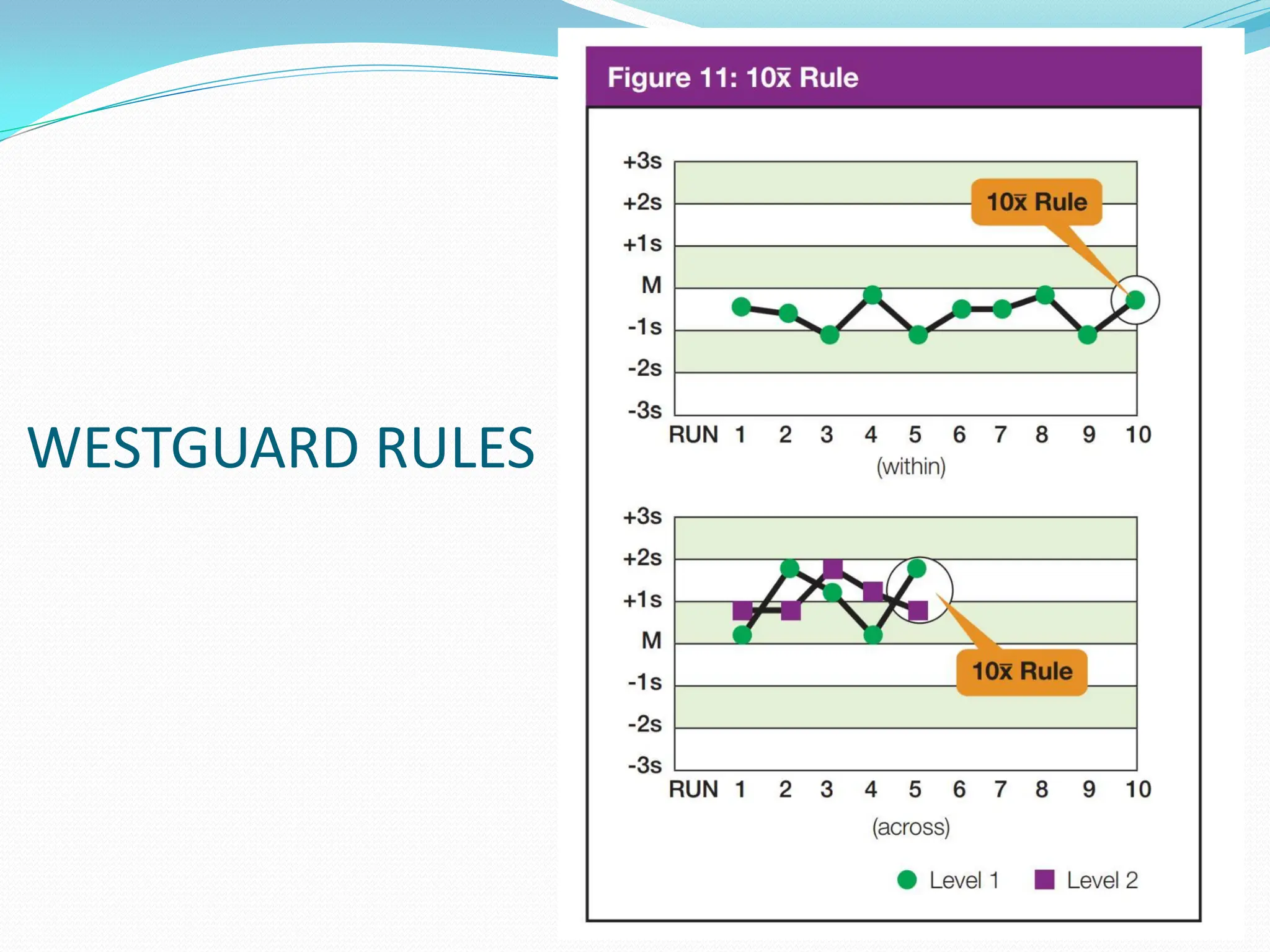
The document outlines an internal quality assurance (QA) program for medical laboratories, detailing its importance in ensuring reliable and clinically useful laboratory test results. It describes components of the QA program, including internal and external quality control processes, and emphasizes the roles of control materials and statistical analysis in validating lab performance. Key concepts discussed include accuracy, precision, random and systemic errors, and procedures for root cause analysis to enhance overall quality assurance efforts.