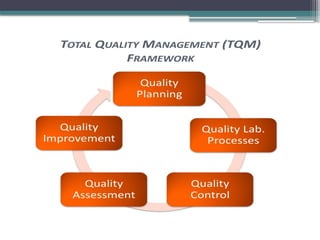
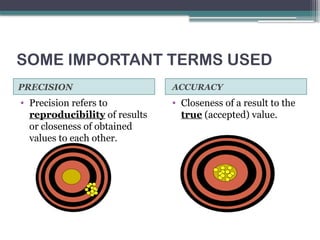
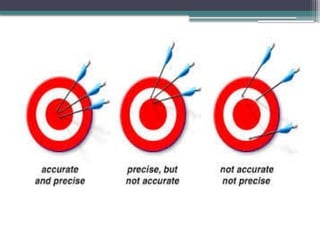
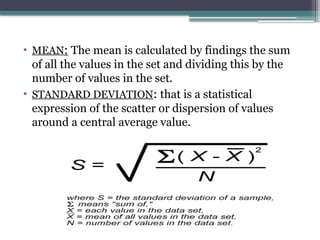
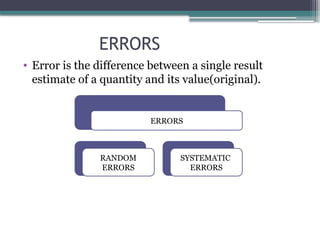
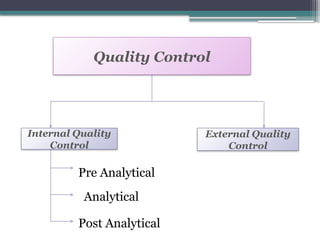
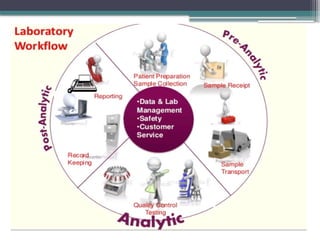
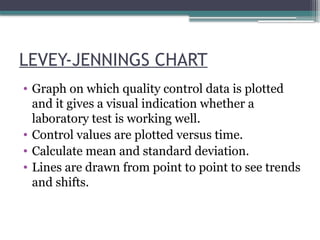
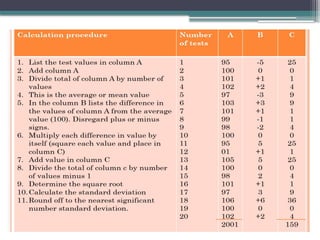
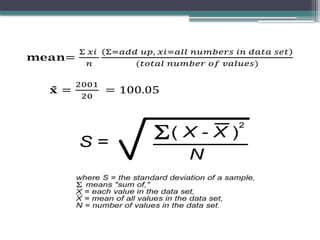
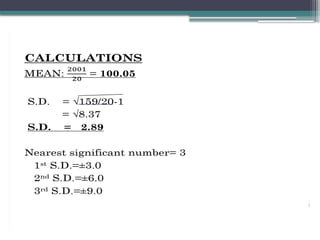
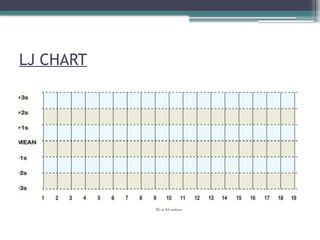
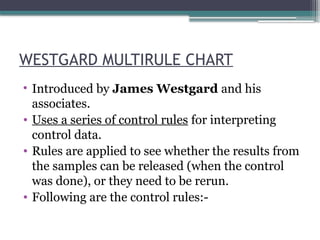
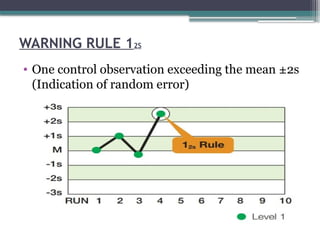
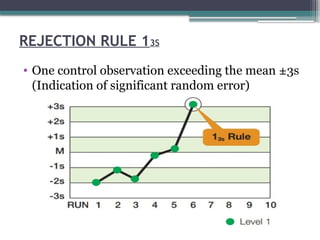
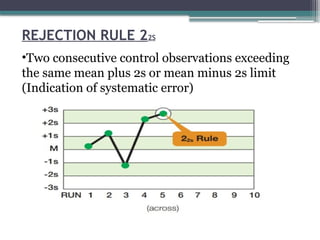
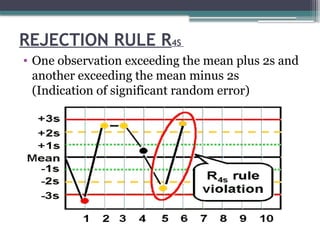
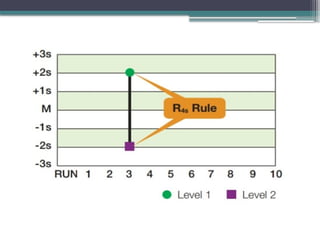
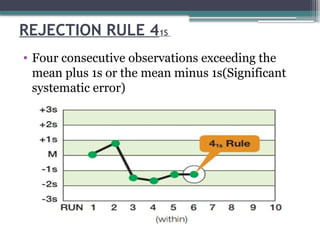
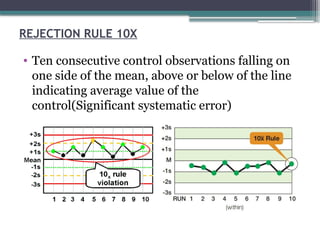
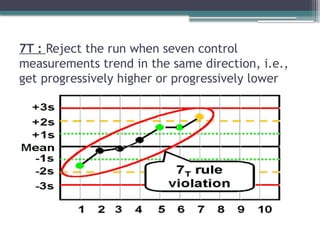
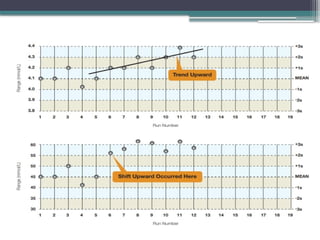
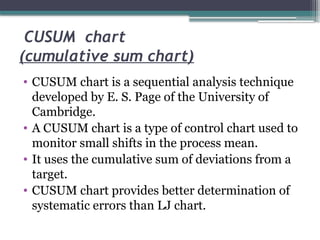
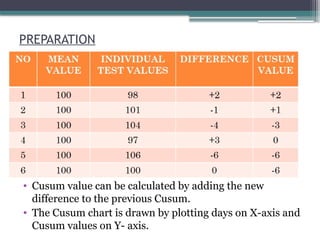
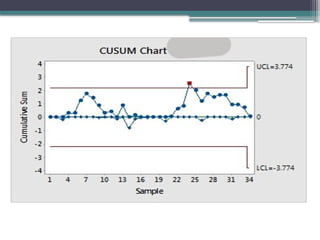
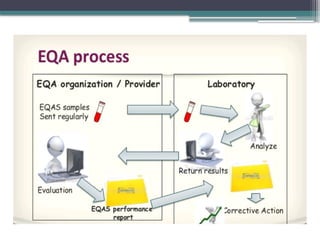
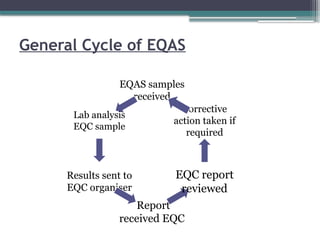
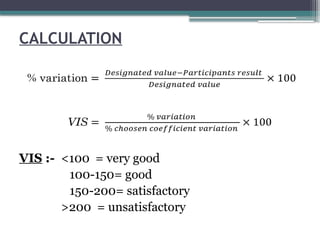
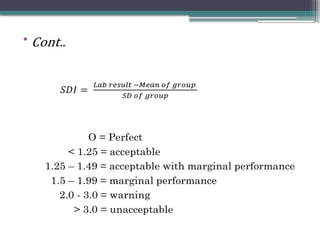
The document discusses quality control in clinical laboratories, emphasizing the importance of meeting customer requirements and ensuring reliable testing through continuous assessment. It outlines internal and external quality control procedures, types of errors, and various methodologies, including control charts, to monitor the quality of laboratory results. Additionally, it highlights the collaborative responsibility of laboratory specialists, clinicians, and public health physicians in maintaining high-quality standards.