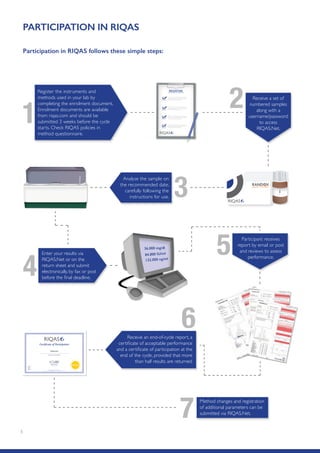
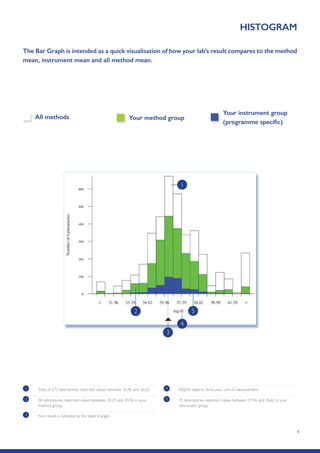
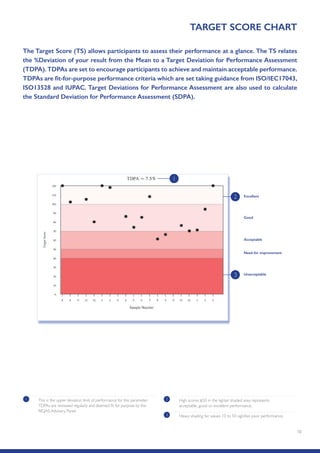
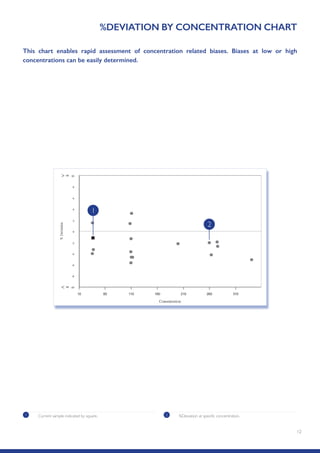
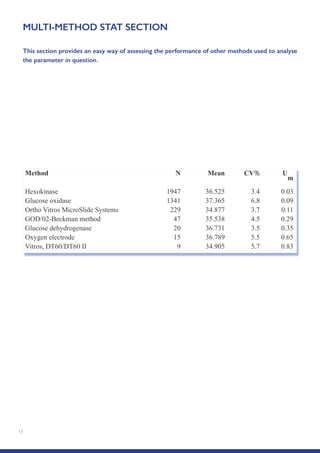
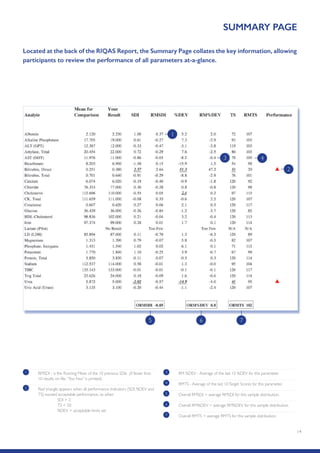
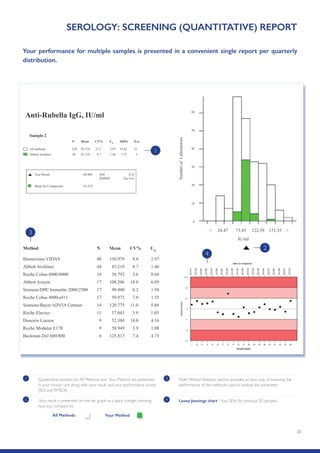
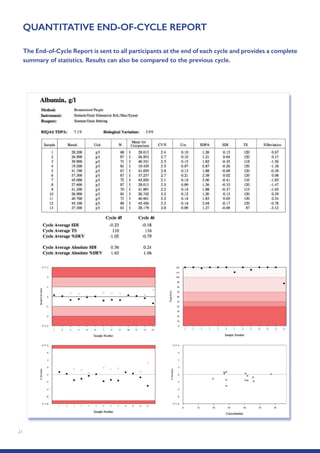
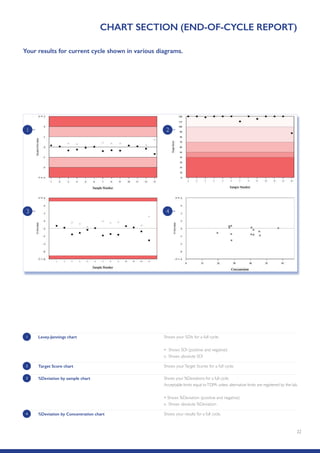
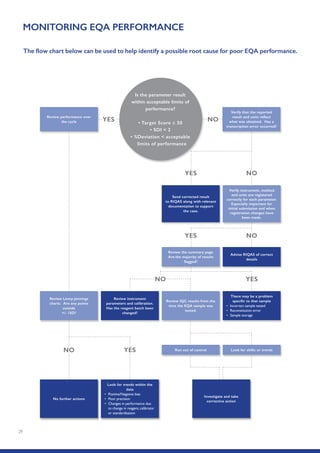
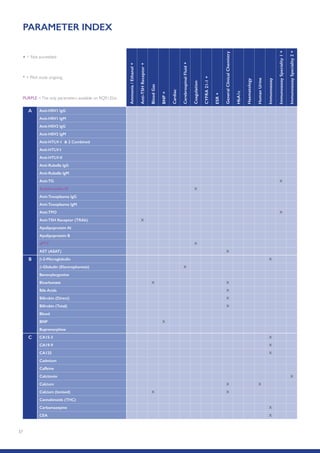
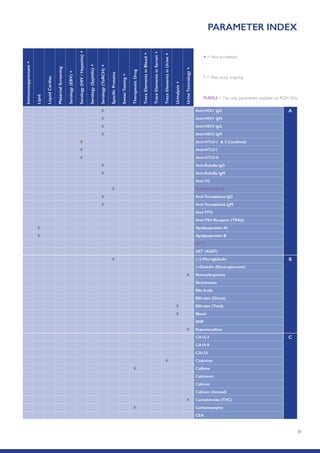
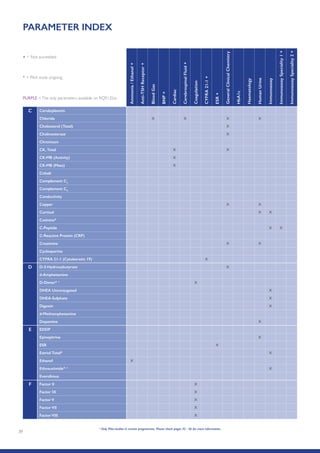
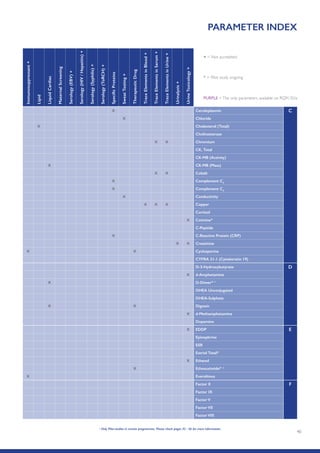
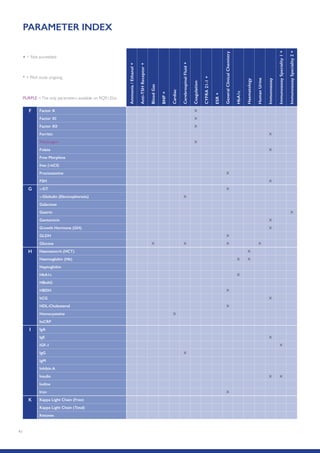
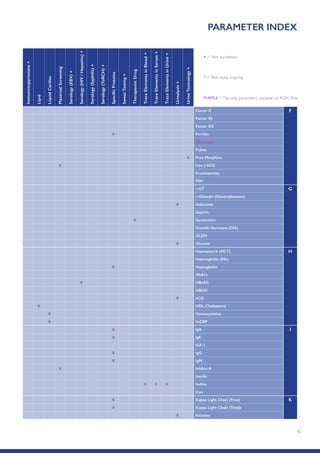
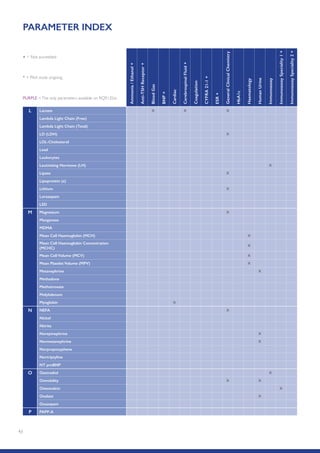
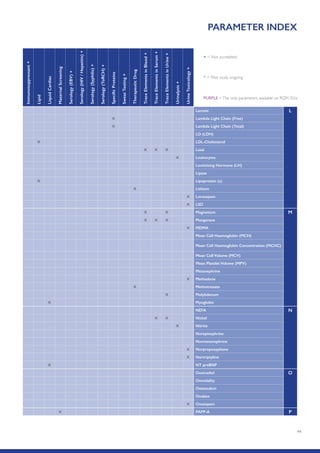
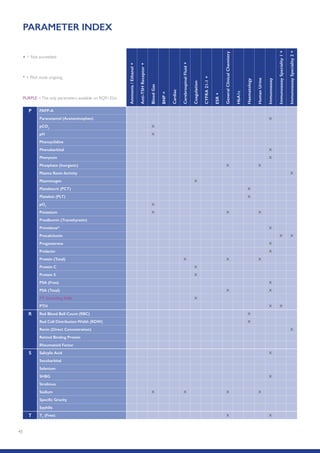
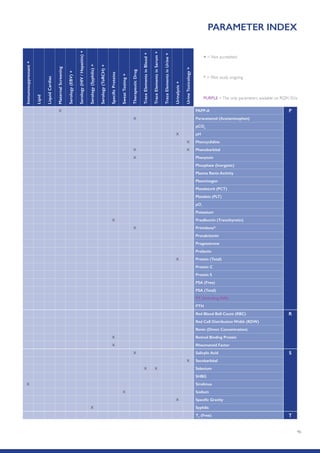
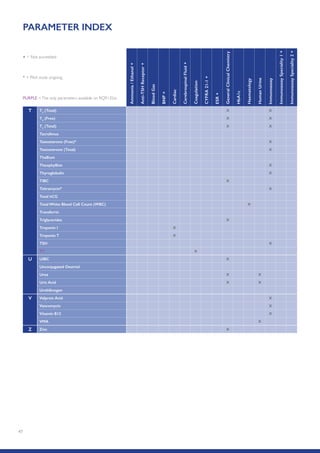
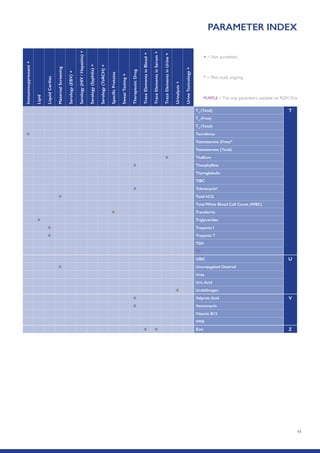
The Randox International Quality Assessment Scheme (RIQAS) is the largest global EQA scheme with over 45,000 laboratory participants, offering a comprehensive solution to enhance test system accuracy and meet regulatory requirements. It provides user-friendly reports with statistical performance data, frequent reporting for early error detection, and is accredited internationally to ensure high quality. Participants benefit from access to a variety of programs and expert support, allowing for effective monitoring of laboratory performance.