1. The document discusses the periodic table, which arranges elements by atomic number and properties. It describes groups and periods of the periodic table.
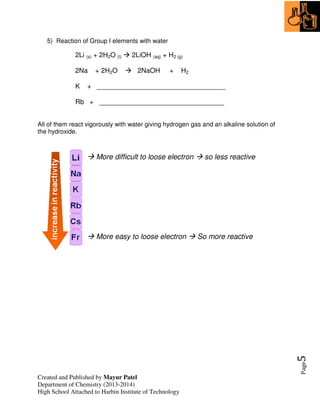
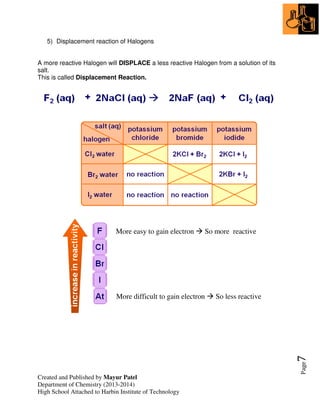
2. Trends in groups 1 (alkali metals) and 7 (halogens) are examined, including their reactivity and properties that change down the groups.
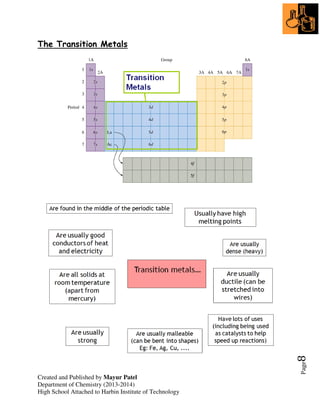
3. Transition metals can have different charges, and are often used as catalysts to increase reaction rates without participating.
4. Noble gases are unreactive as they have no tendency to gain or lose electrons.