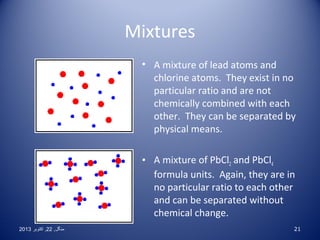
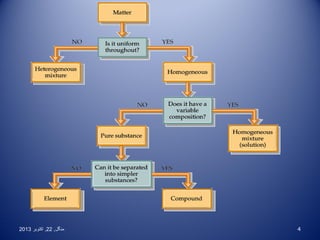
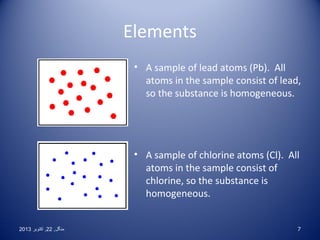
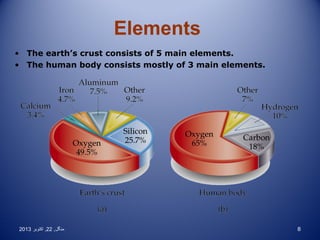
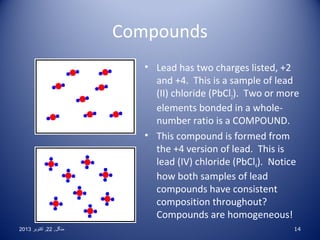
This document discusses the classification and properties of elements, compounds, and mixtures. It defines elements as pure substances made of only one type of atom that cannot be broken down further. Compounds are also pure substances with a fixed composition made of two or more elements chemically bonded together. Mixtures are impure substances that can have varying compositions and whose components can be separated physically. Compounds and elements are homogeneous, while mixtures can be either homogeneous or heterogeneous. The document provides many examples and characteristics to distinguish between these three classifications of matter.










![Types of Compounds
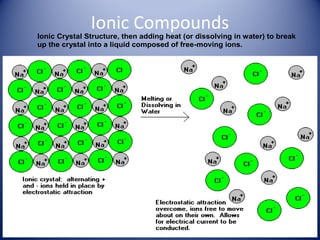
• Ionic: made of metal and nonmetal ions. Form an ionic
crystal lattice when in the solid phase. Ions separate
when melted or dissolved in water, allowing electrical
conduction. Examples: NaCl, K2O, CaBr2
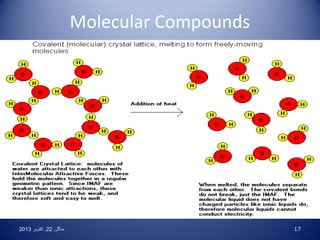
• Molecular: made of nonmetal atoms bonded to form a
distinct particle called a molecule. Bonds do not break
upon melting or dissolving, so molecular substances do
not conduct electricity. EXCEPTION: Acids [H+A- (aq)]
ionize in water to form H3O+ and A-, so they do conduct.
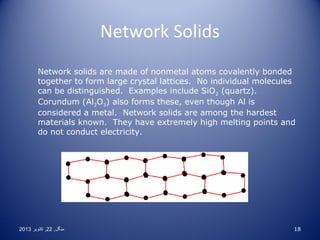
• Network: made up of nonmetal atoms bonded in a
seemingly endless matrix of covalent bonds with no
distinguishable molecules. Very high m.p., don’t conduct.
15
2013 منگل , 22 , اکتوبر](https://image.slidesharecdn.com/elementscompoundsandmixtures-131021203856-phpapp02/85/Elements-compounds-and-mixtures-15-320.jpg)