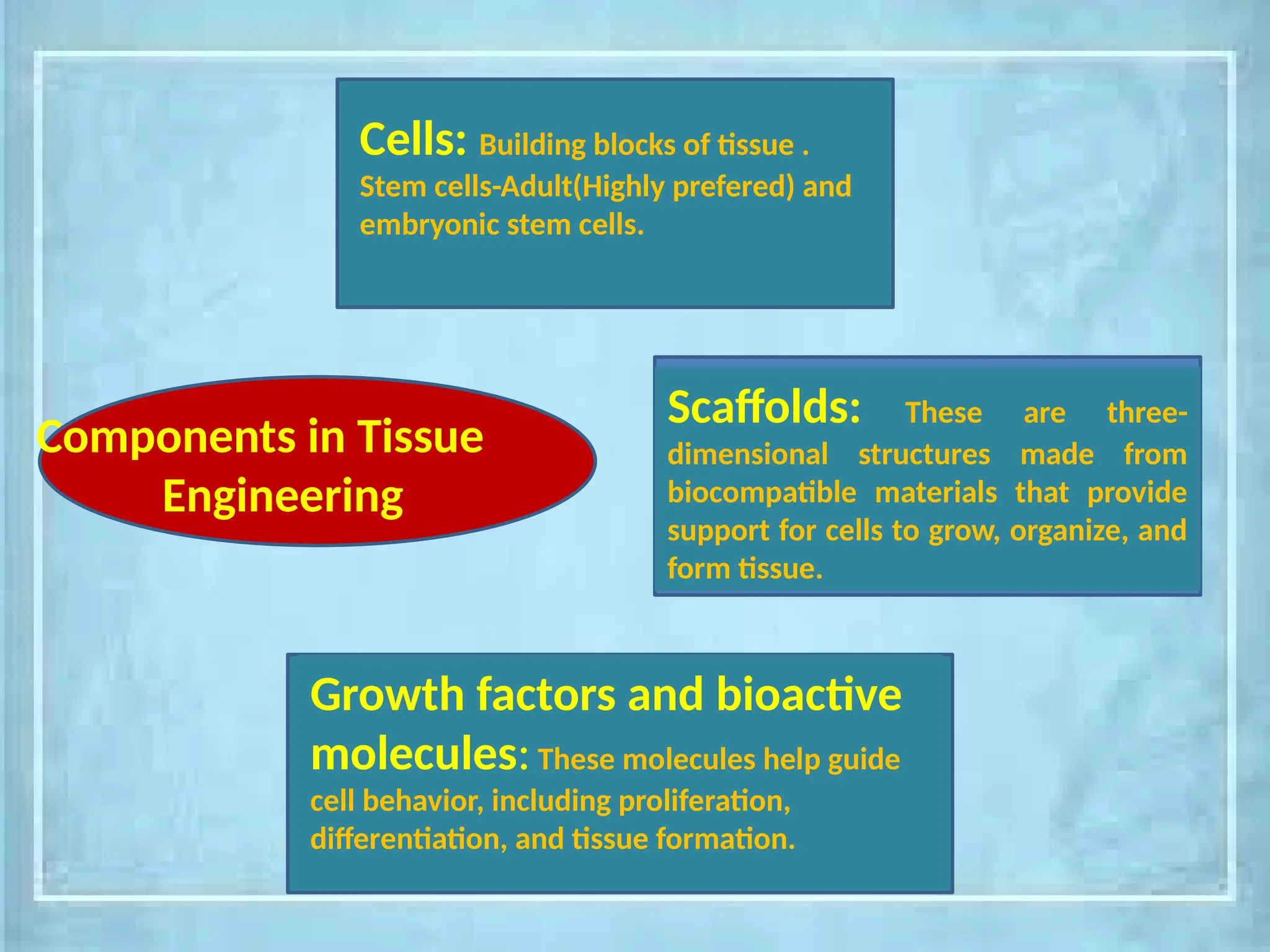
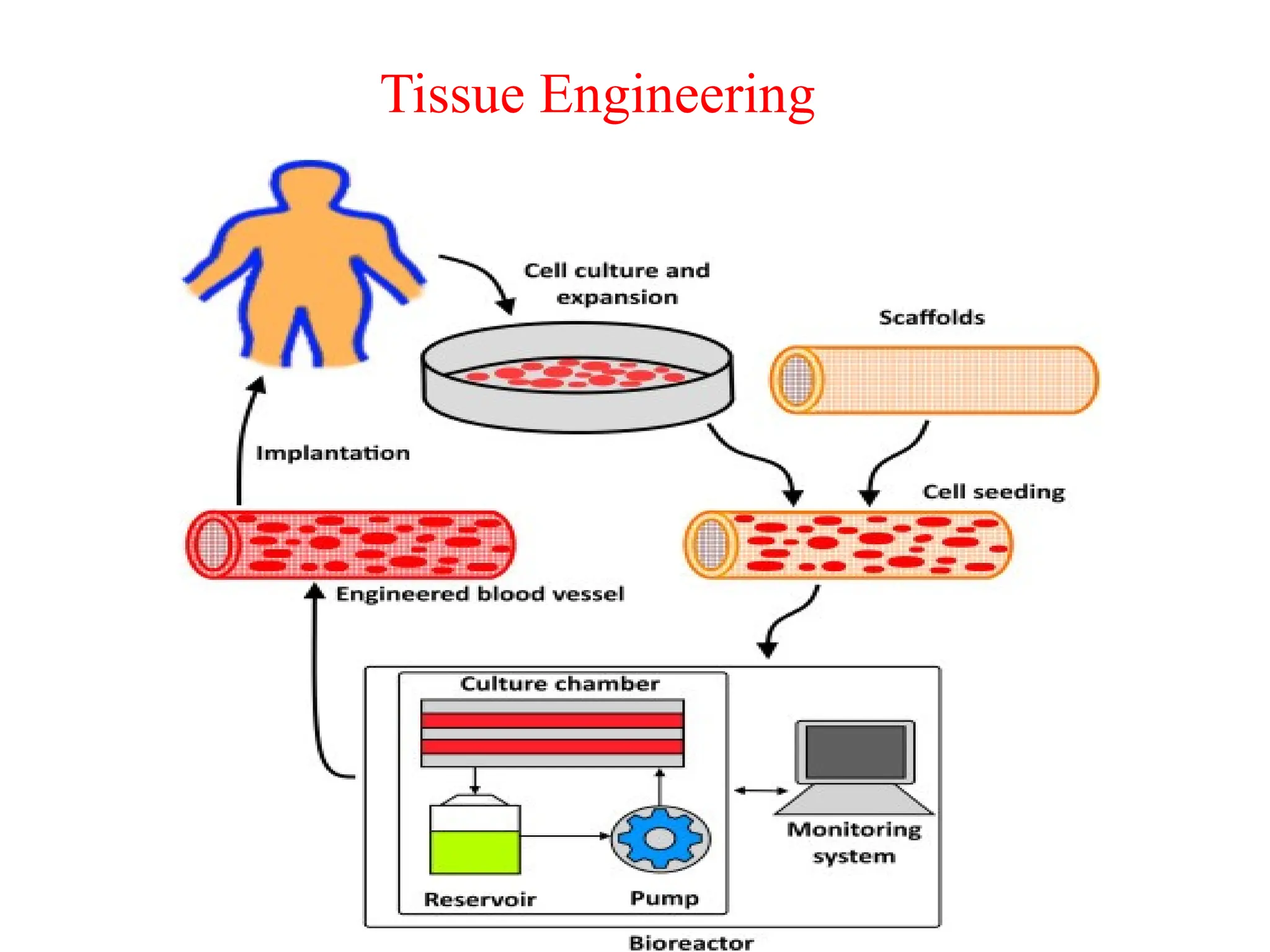
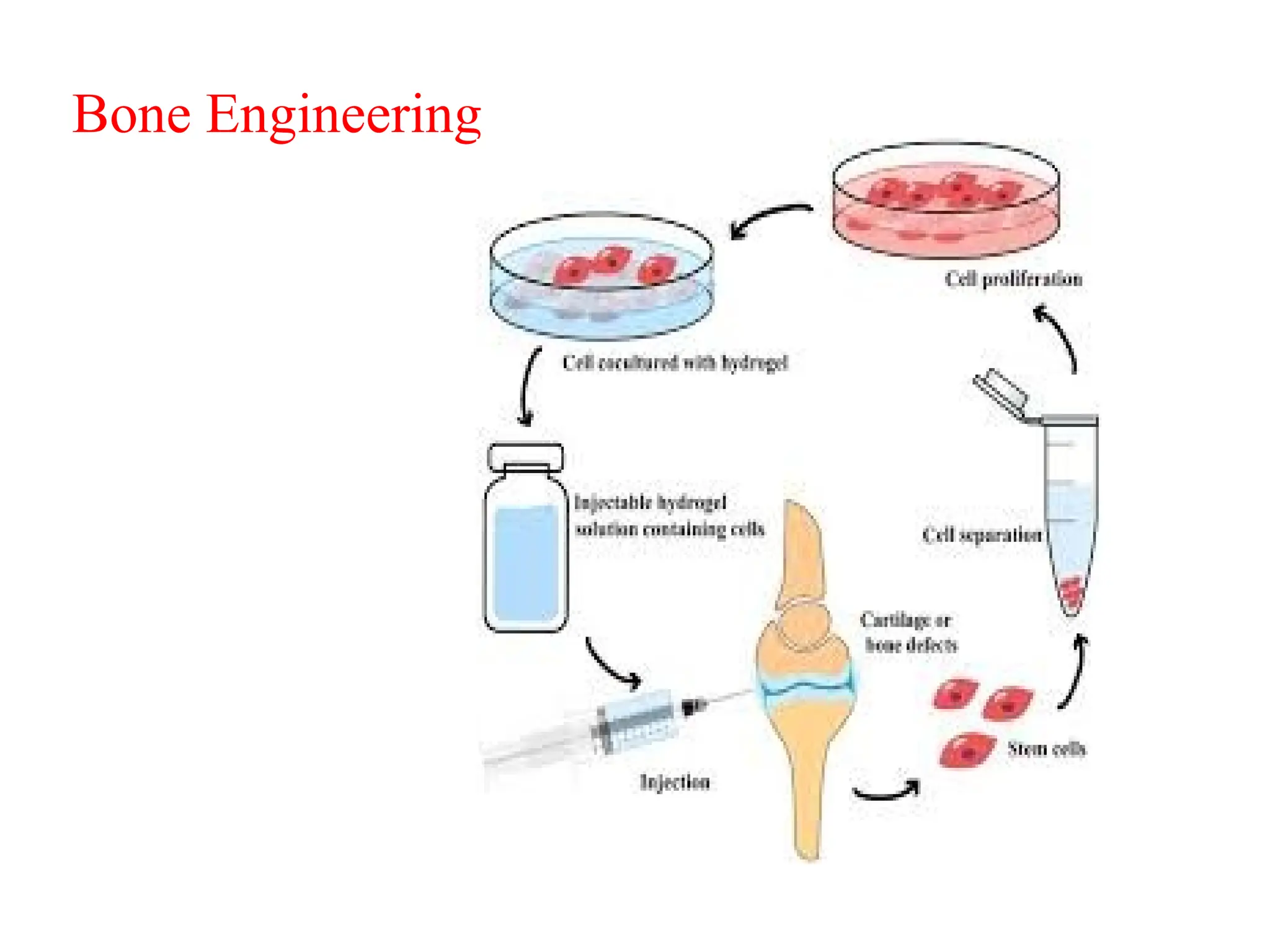
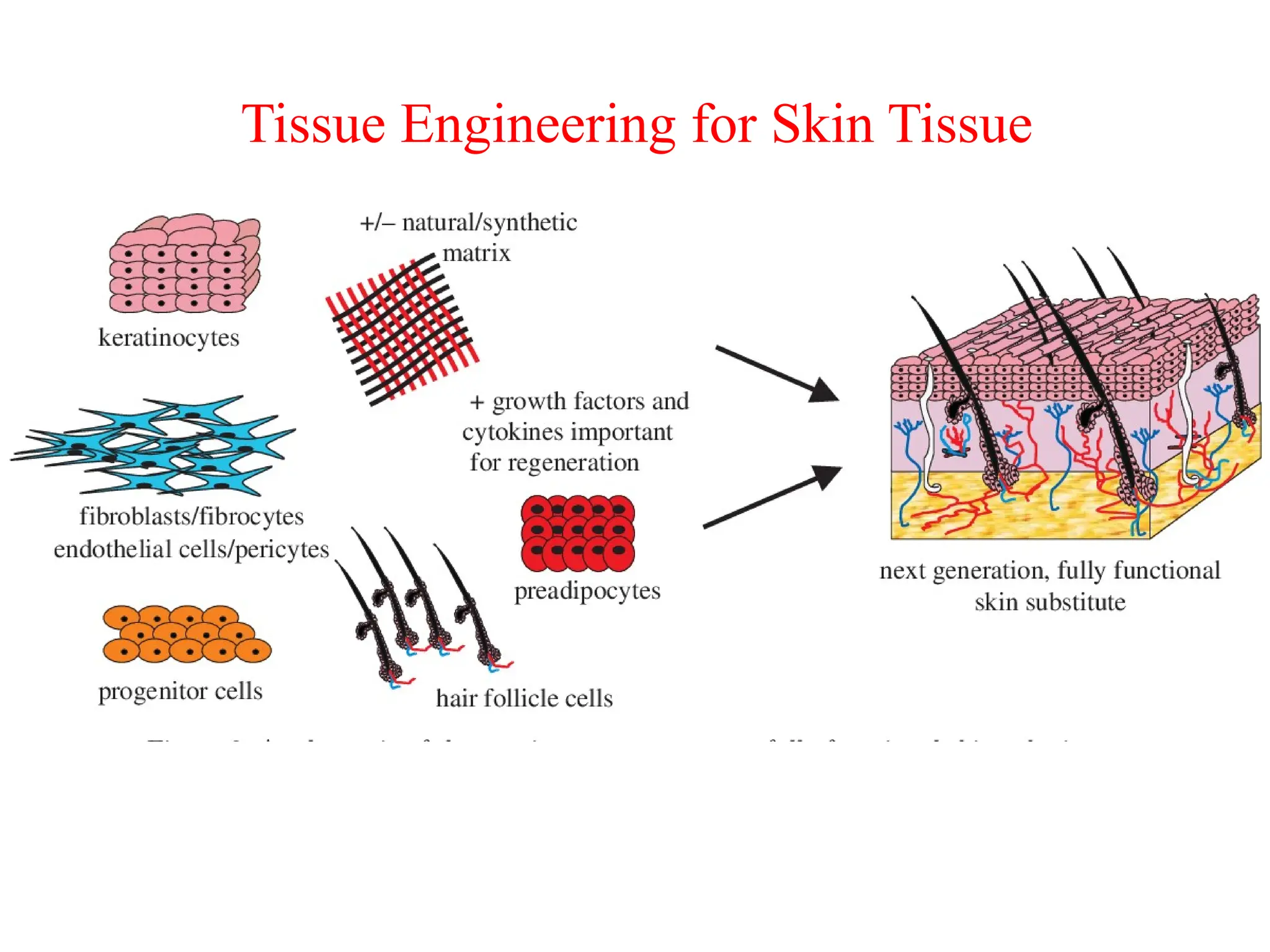
The document provides an overview of tissue engineering, focusing on its applications in bone, skin, and neural tissues. It details the essential components such as cells, scaffolds, and growth factors involved in engineering functional tissues, highlighting methods like 3D bioprinting and bioreactors to improve the efficacy of tissue constructs. Key considerations for each type of tissue engineering, including specific cell types and biomaterials, are also discussed.