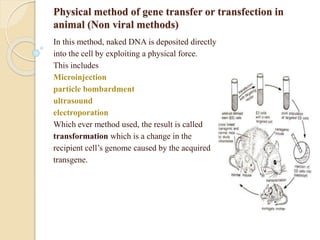
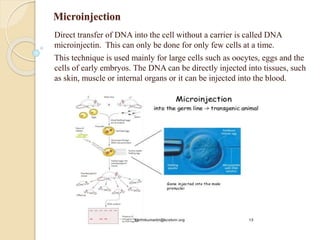
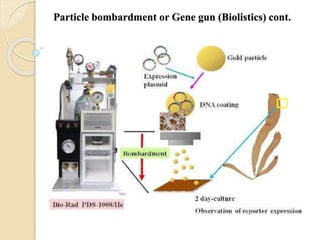
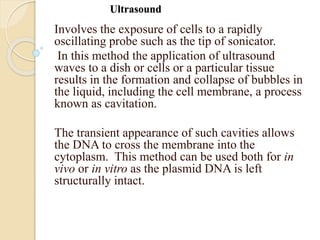
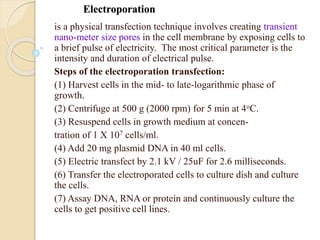
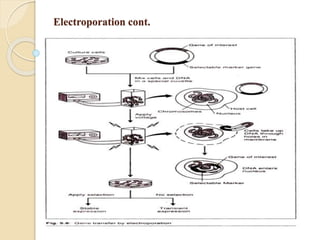
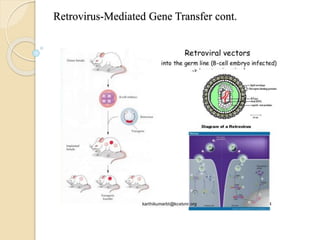
This document discusses various methods for transferring genes into animal cells, including viral and non-viral approaches. Viral methods use viruses like adenovirus to transfer genes, while non-viral methods include biochemical techniques like calcium phosphate transfection, lipid-mediated transfection using lipofectamine, and physical methods like microinjection, particle bombardment/gene guns, ultrasound, and electroporation. The document provides detailed protocols for lipid-mediated transfection and some of the other non-viral methods.



![Lipid mediated method
This method can be used for both transient and stable
transfection, and it can be used for adherent cells,
primary cell lines, and suspension cultures.
For the following protocol, the Lipofectamine
Reagent from Invitrogen Corporation will be used.
Lipofectamine Reagent is a 3:1 (w/w)
Liposome formulation of the plycationic lipid 2,3-
dioleyloxy-N-[2(sperminecardox-ido)ethyl]-N,N-
dimethyl-1-propanaminium trifluoro-acetate
(DOSPA) and the neutral lipid dioleoyl
phosphatidylethanolamine (DOPE) in membrane-
filtered water.](https://image.slidesharecdn.com/genetransferinanimals-180303065418/85/Gene-transfer-in-animals-8-320.jpg)