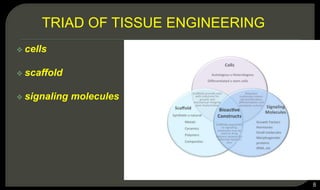
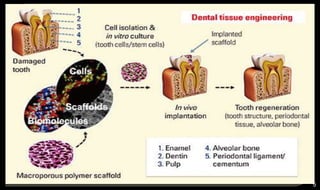
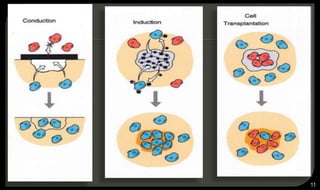
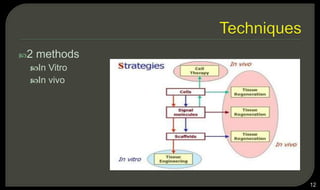
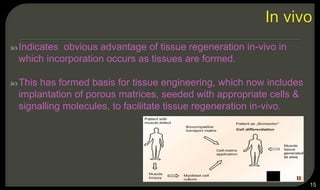
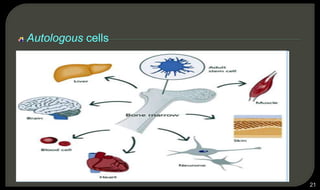
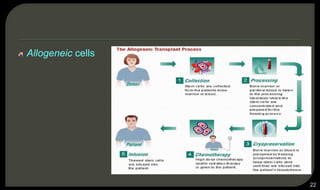
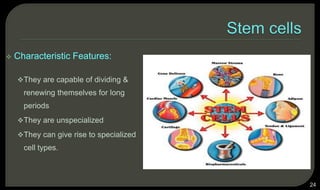
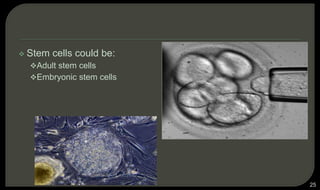
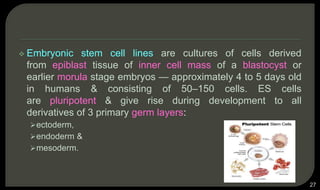
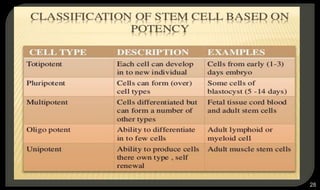
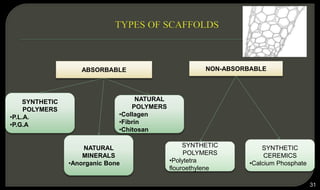
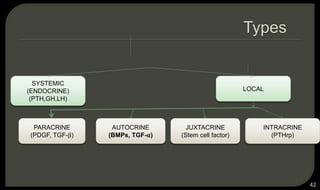
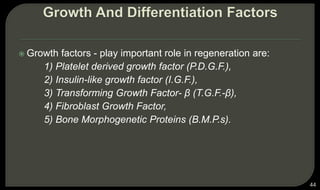
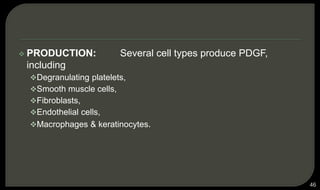
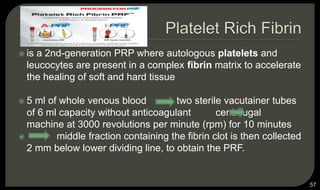
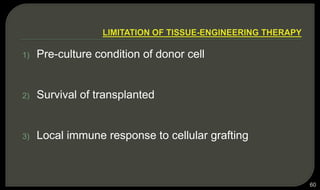
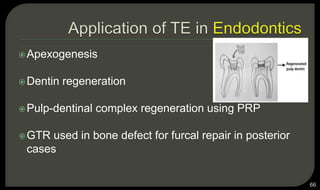
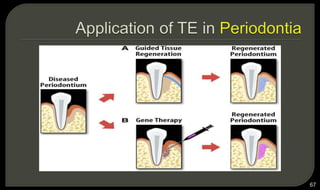
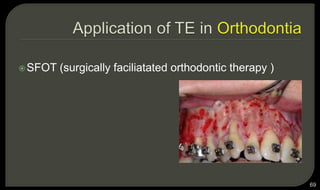
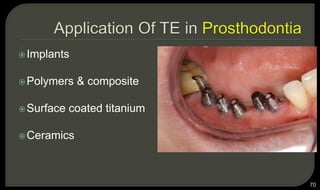
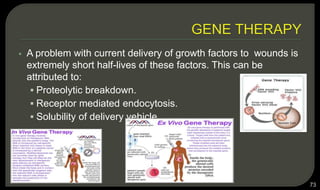
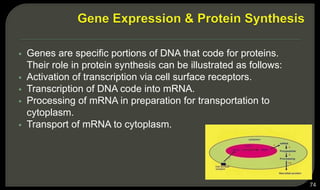
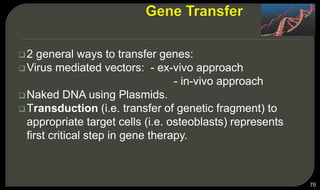
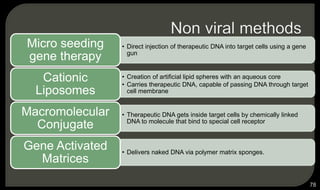
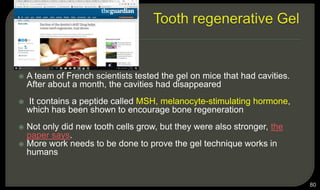
This document provides an overview of tissue engineering presented by Dr. Boris Saha. It defines tissue engineering as combining principles of life sciences and engineering to develop materials and methods to repair damaged tissues. The key elements of tissue engineering are discussed as cells, scaffolds, and signaling molecules. Various cell types, scaffold materials, and growth factors used in tissue engineering are described. Techniques for tissue engineering include both in vitro and in vivo approaches. Limitations and future perspectives of tissue engineering are also mentioned.