Embed presentation


![Reaction through associative mechanism
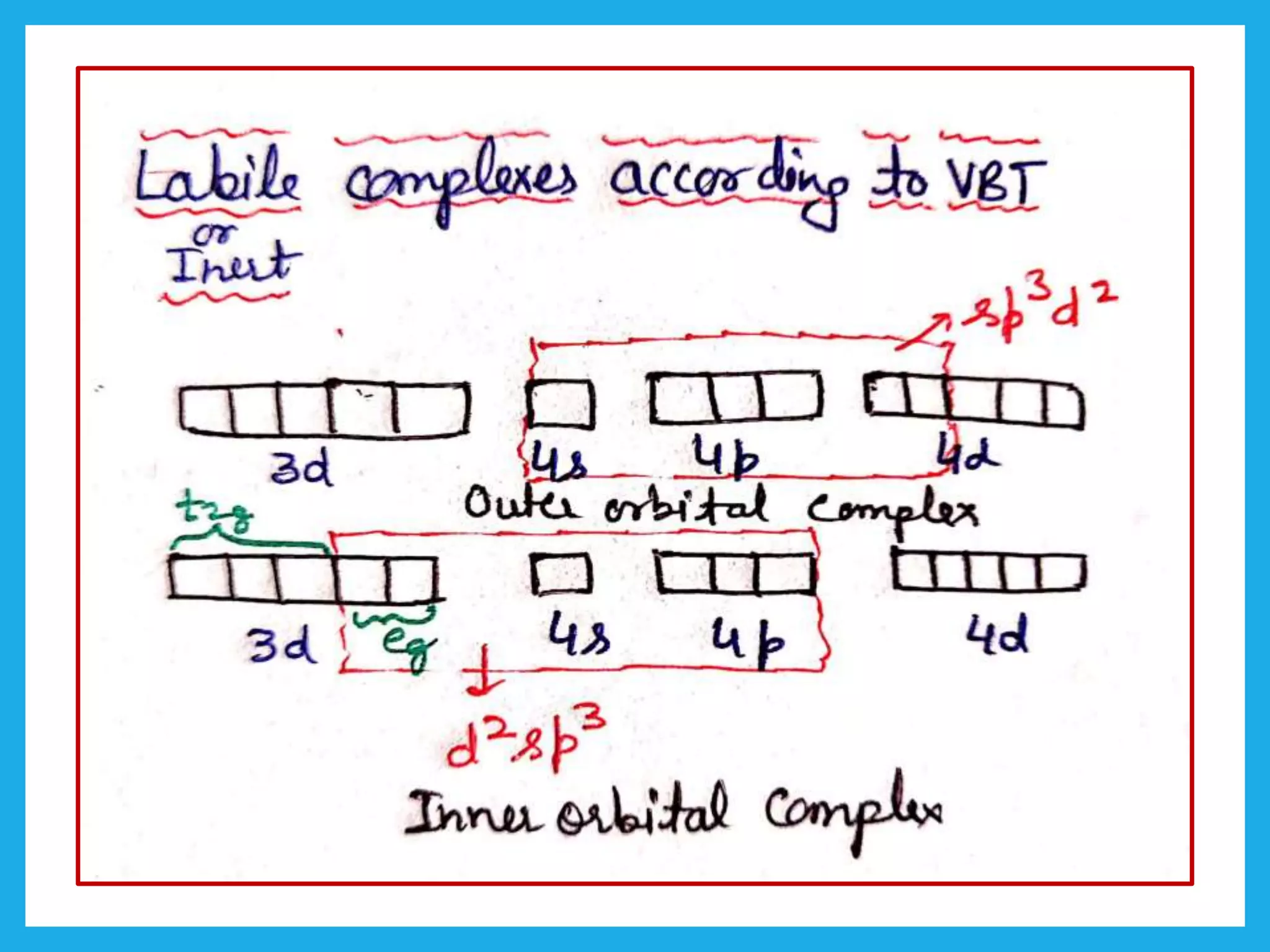
Outer orbital and inner orbital complexes with low lying
unhybridized metal d-orbitals.
These orbitals are center attack by the incoming ligand in
associated mechanism of substitution.
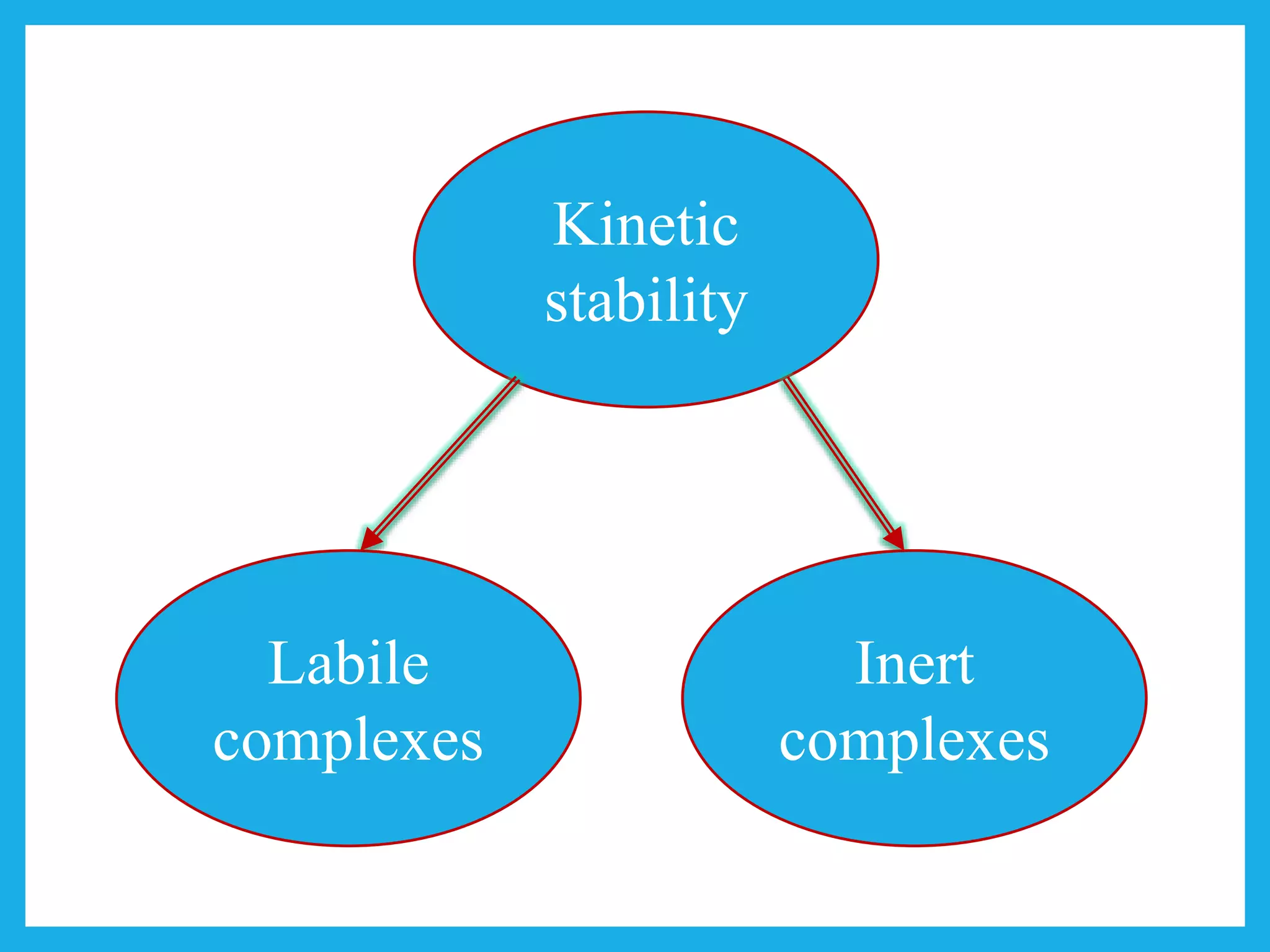
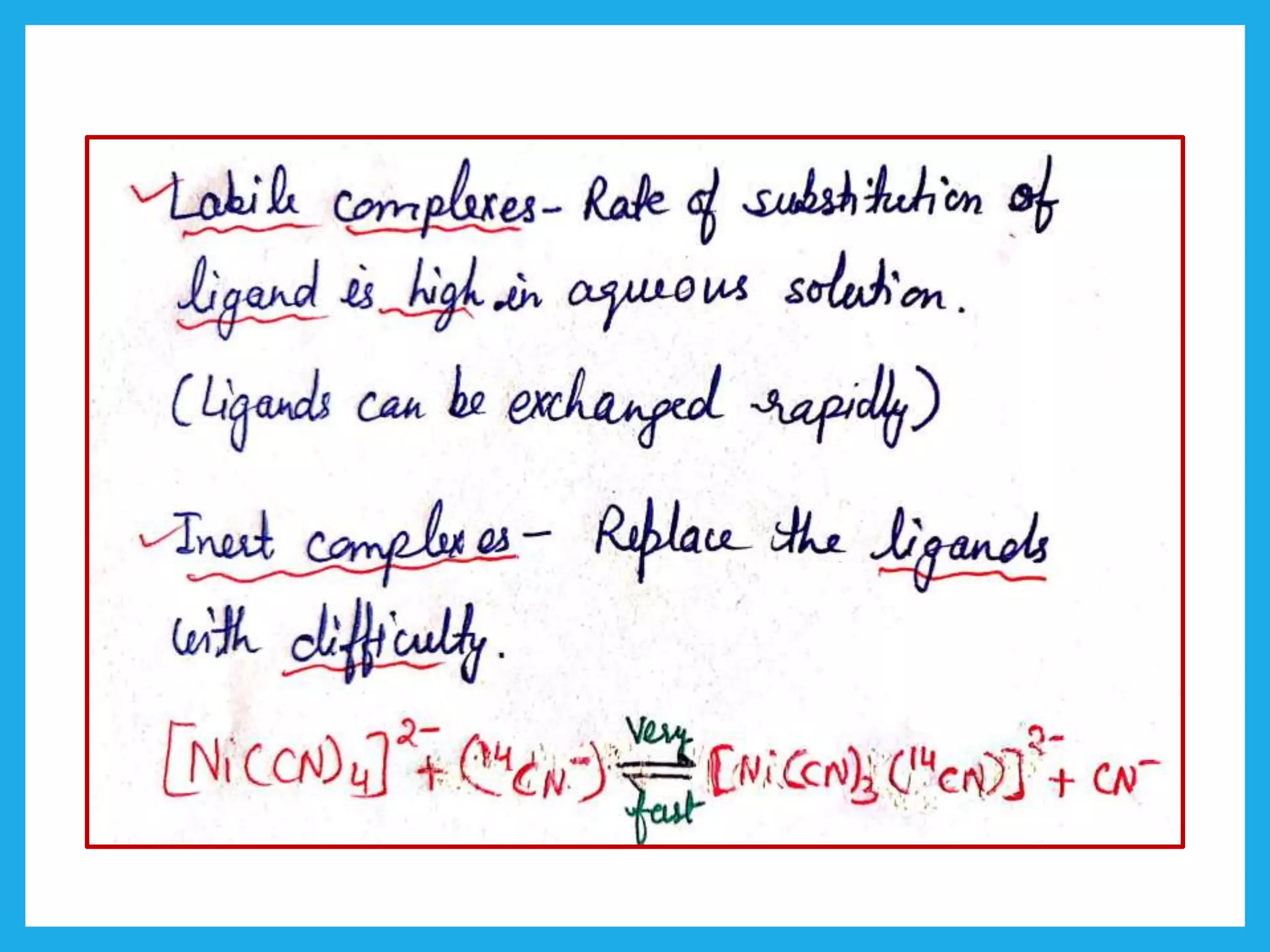
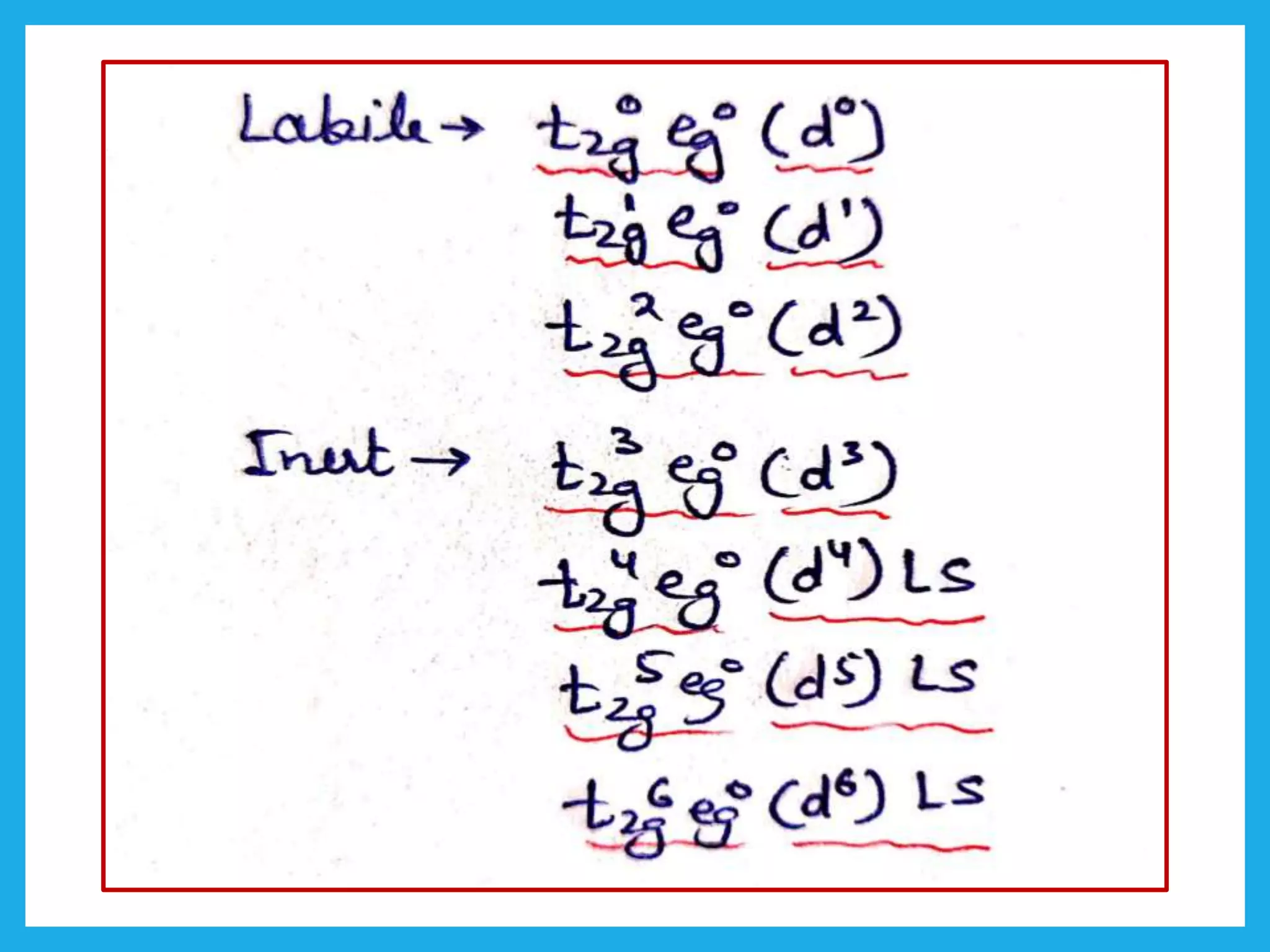
Inert complexes
Electrons are present in the unhybridized d-orbitals.
Labile complexes
Vacant unhybridized d-orbitals.
[MnCl6]3- = Labile
[Cr(CN)6]3- = Inert
d2sp3
XX XX XX XX XX XX
XX XX XX XX XX XX
sp3d2](https://image.slidesharecdn.com/11thermodynamicandkineticstabilitypart2kineticstability-230424025759-755bb814/75/Thermodynamic-and-kinetic-stability-Part-2-kinetic-stability-pptx-6-2048.jpg)





This document discusses the kinetic stability of metal complexes through dissociative and associative reaction mechanisms. Inner orbital complexes with d2sp3 hybridization form shorter, stronger metal-ligand bonds and are always inert. Outer orbital complexes with sp3d2 hybridization form larger, weaker bonds and can be either labile or inert depending on the mechanism. For the associative mechanism, complexes with low-lying unhybridized d-orbitals can be either inert or labile depending on whether the d-orbitals are occupied or vacant. The lability is determined by calculating the change in crystal field stabilization energy between the transition state and original complex.





![Reaction through associative mechanism
Outer orbital and inner orbital complexes with low lying
unhybridized metal d-orbitals.
These orbitals are center attack by the incoming ligand in
associated mechanism of substitution.
Inert complexes
Electrons are present in the unhybridized d-orbitals.
Labile complexes
Vacant unhybridized d-orbitals.
[MnCl6]3- = Labile
[Cr(CN)6]3- = Inert
d2sp3
XX XX XX XX XX XX
XX XX XX XX XX XX
sp3d2](https://image.slidesharecdn.com/11thermodynamicandkineticstabilitypart2kineticstability-230424025759-755bb814/75/Thermodynamic-and-kinetic-stability-Part-2-kinetic-stability-pptx-6-2048.jpg)





