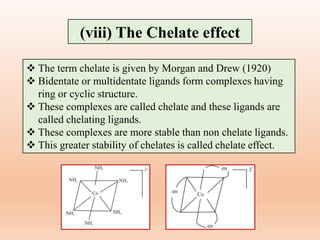
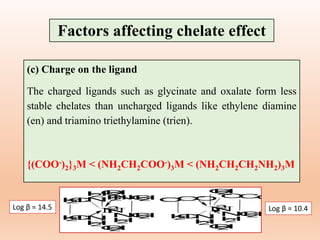
The document discusses factors that affect the stability of metal complexes. It outlines three main factors: (1) the nature of the central metal ion, (2) the nature of the ligand, and (3) surrounding conditions. For the nature of the ligand, it discusses several sub-factors that can influence stability, including size and charge of the ligand, its basic character, concentration, covalent character, and ability to form chelate complexes or use resonance. The document provides examples to illustrate each factor and sub-factor. It also discusses applications of chelate complexes.






![(iii) Ligand concentration
When SCN- (thiocynate ion) ligand is present in the solution
in high amount, the Co2+ metal ion forms a stable blue
colored complex [Co(SCN)4]2- with SCN- while on dilution,
the blue coloured complex destroyed and a pink complex
[Co(H2O)6]2+ is formed.
Further addition of SCN- ligand again regenerates blue
coloured complex and destroyed pink colored complex.
Competition between the two i.e. H2O and SCN- for
coordination with the cobalt ion is indicated by the colour
change.
[Co(SCN)4]2- + 6H2O [Co(H2O)6]2+ + 4SCN-
Blue Pink](https://image.slidesharecdn.com/14thermodynamicandkineticstabiltypart5-230424030317-1516194b/85/Thermodynamic-and-kinetic-stability-Part-5-pptx-7-320.jpg)
![During synthesis of tetra amine cupric sulphate complex
[Cu(NH3)4SO4.H2O], at lower concentration of ammonia, copper
hydroxide is formed, while at higher concentration of ammonia,
tetra amine cupric sulphate complex is formed.
CuSO4 + NH4OH (low concentration) → Cu(OH)2
CuSO4 + NH4OH (high concentration) → [Cu(NH3)4SO4.H2O]
(iii) Ligand concentration](https://image.slidesharecdn.com/14thermodynamicandkineticstabiltypart5-230424030317-1516194b/85/Thermodynamic-and-kinetic-stability-Part-5-pptx-8-320.jpg)






![(b) Number of rings
The chelates in which the polydentate ligand
form more number of cyclic or ring structure is
more stable than the chelate in which a
polydentate ligand form lower number of ring
structures.
EDTA (Ethylene diamine tetraacetic acid)
complex having five chelate rings, is more
stable than complex with four monodentate
carboxylate ligands and two monodentate
nitrogen donor ligands.
Factors affecting chelate effect
[Ca(EDTA)]2-](https://image.slidesharecdn.com/14thermodynamicandkineticstabiltypart5-230424030317-1516194b/85/Thermodynamic-and-kinetic-stability-Part-5-pptx-16-320.jpg)
![• The complex [Ni(dien)2)]2+ is more stable (4 rings) than the
complex [Ni(en)3)]2+ (3 rings).
• Both the complexes are octahedral with six nitrogen atoms
surrounding the nickel ion.
• dien (diethylenetriamine, 1,4,7-triazaheptane) is
a tridentate ligand and en is a bidentate ligand.
Factors affecting chelate effect
H 2 N
N
H
N H 2
N i
N H 2
H 2 C
H 2 C
N H
H 2 C
C H 2
N H 2
H N C H 2
C H 2
H 2 N
C H 2
C
H 2
H 2 N
2 +
N
i
H
2
C
C
H
2
H
2
N
H
2
N
C
H
2
C
H
2
N
H
2
N
H
2
H
2
C
H
2
C
N
H
2
N
H
2
2
+](https://image.slidesharecdn.com/14thermodynamicandkineticstabiltypart5-230424030317-1516194b/85/Thermodynamic-and-kinetic-stability-Part-5-pptx-17-320.jpg)

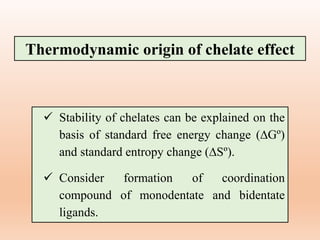
![[Cd(H2O)4]2+ + 4NH3 → [Cd(NH3)4]2+ + 4H2O.......i, ∆Hº=-ve
Monodentate ligand Non-chelated compound
1 molecule 4 molecules 1 molecule 4 molecules
Δn1 = 0; ΔS1 = 0
[Cd(H2O)4]2+ + 2en → [Cd(en)2]2+ + 4H2O.......ii, ∆Hº=-ve
Bidentate ligand Chelated compound
1 molecule 2 molecules 1 molecule 4 molecule
n2 = 2; S2 = +ve
[Cd (H2O)4]2+ + trien Cd (trien)]2+ + 4H2O....ii, ∆Hº=-ve
Tridentate ligand Chelated compound
1 molecule 1 molecules 1 molecule 4 molecule
n2 = 3; S3 = +ve](https://image.slidesharecdn.com/14thermodynamicandkineticstabiltypart5-230424030317-1516194b/85/Thermodynamic-and-kinetic-stability-Part-5-pptx-21-320.jpg)
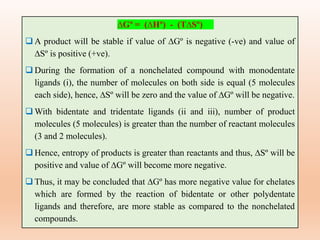




![Many complexes that are stable under specific
conditions may not be stable under some other set of
conditions. [Co(NH3)6]3+ complex is stable in
aqueous solution under neutral conditions while
unstable in an acidic solution.
[Co(NH3)6]3+ + 6H3O+ → [Co(H2O)6]3+ + 6NH4
+
Hence, stability of a complex should be discussed
with the surrounding conditions such as heat, light,
acidity or basicity.
(3) Surrounding conditions](https://image.slidesharecdn.com/14thermodynamicandkineticstabiltypart5-230424030317-1516194b/85/Thermodynamic-and-kinetic-stability-Part-5-pptx-27-320.jpg)
