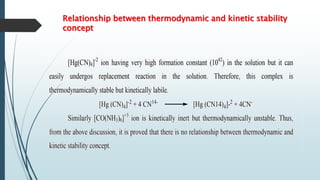
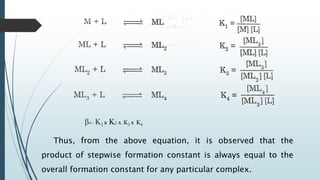
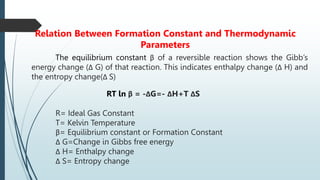
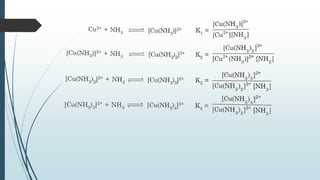
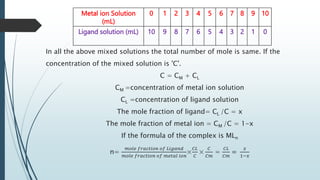
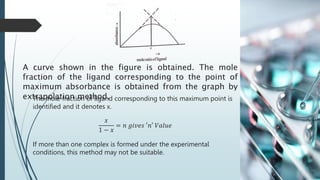
The document discusses the stability of metal complexes, focusing on both thermodynamic and kinetic stability concepts. It outlines factors affecting stability, including the nature of the central metal ion and ligands, as well as the crystal field stabilization energy. Additionally, it describes methods for determining the composition of metal complexes, particularly Job's method using spectrophotometric techniques.