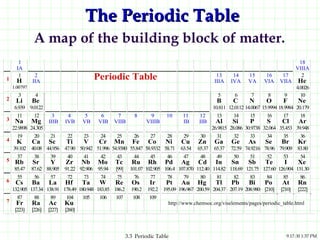
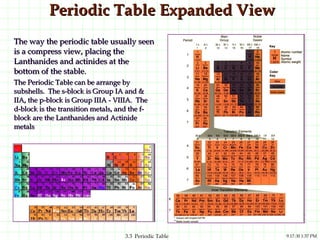
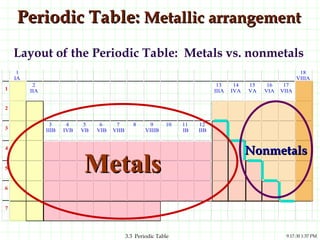
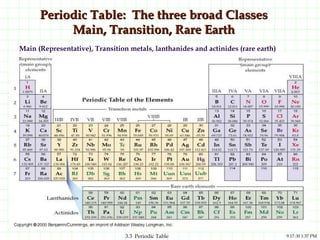
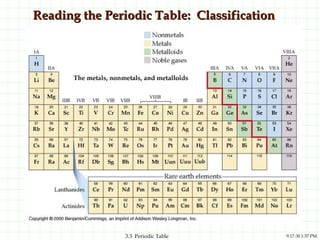
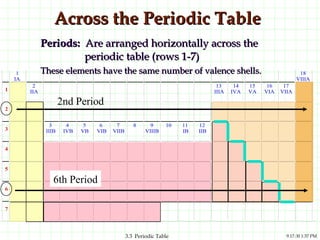
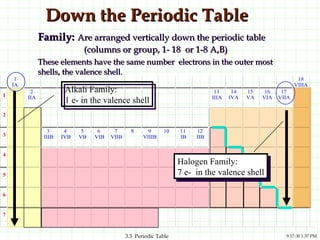
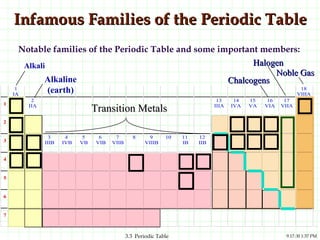
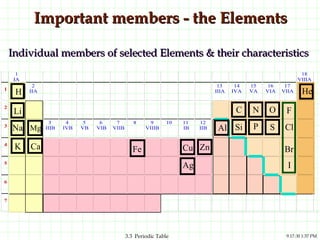
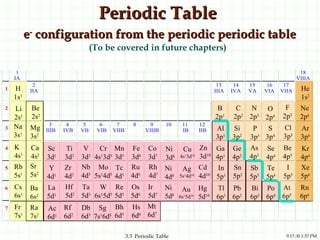
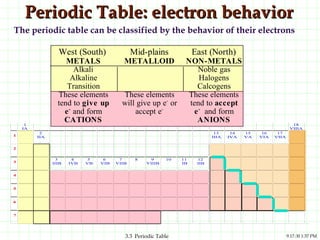
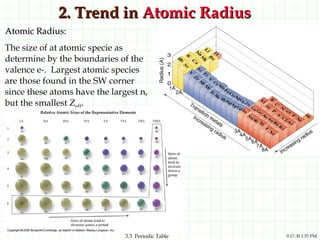
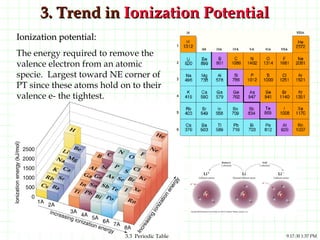
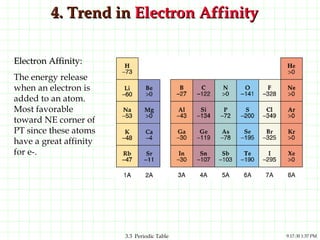
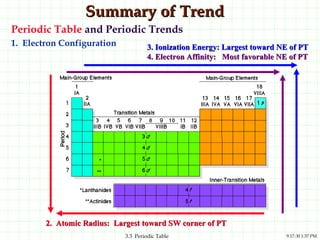
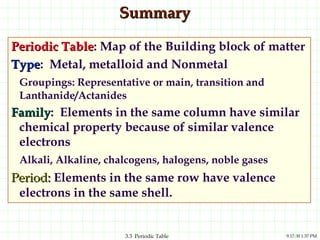
The document discusses the periodic table and periodic trends. It explains that the periodic table organizes the elements based on their atomic structure and properties. Elements are arranged in rows and columns, with elements in the same column having similar chemical properties due to their valence electrons. The document outlines trends in atomic radius, ionization energy, and electron affinity across the periodic table, noting that these properties are greatest for elements in the northeast corner which hold their valence electrons most tightly.