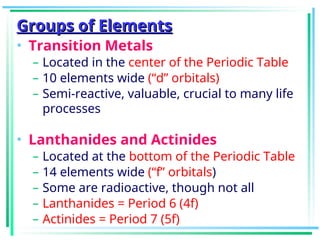
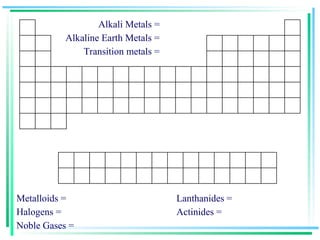
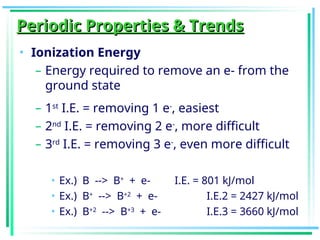
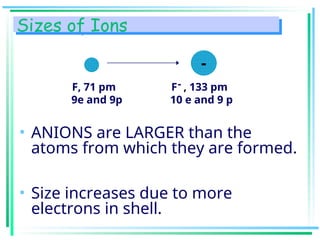
The document outlines the history and development of the periodic table, highlighting key contributions from various scientists such as Antoine Lavoisier, Johann Döbereiner, and Dmitri Mendeleev. It discusses the modern periodic law, element classifications, and periodic trends including atomic radius, ionization energy, and electronegativity. Additionally, it explains the characteristics of different groups of elements and their reactivity, providing a comprehensive overview of the periodic table's structure and significance in chemistry.

![Reading the Periodic Table
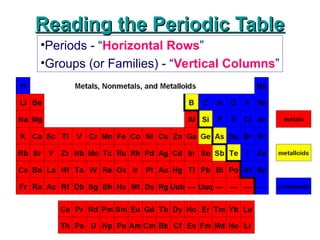
Reading the Periodic Table
• Valence electrons are periodic!
• Notice the similarities
– Ex.) Write the noble gas configurations for:
• F [He]2s2
2p5
7 valence electrons
• Cl [Ne]3s2
3p5
7 valence electrons
• Br [Ar]4s2
3d10
4p5
7 valence electrons
• I [Kr]5s2
4d10
5p5
7 valence electrons
– GROUPS have similar valence electron
configurations!](https://image.slidesharecdn.com/pp-unit10notesperiodictable1-241203061001-07ba9898/85/PP-Unit-10-Notes_Periodic-Table-1-ppt-11-320.jpg)