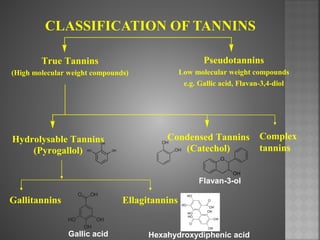
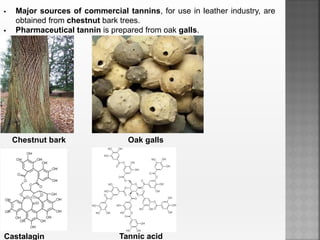
Tannins are high molecular weight phenolic compounds that can precipitate proteins. They are classified as hydrolysable tannins, condensed tannins, and complex tannins. Tannins are found in plants and have properties such as astringency. They have various medicinal uses and are important economically for uses like leather tanning.