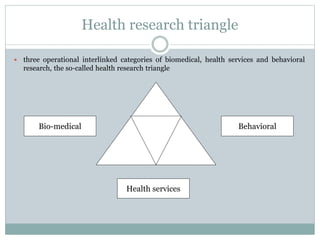
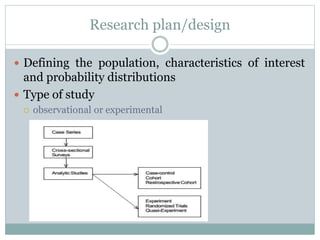
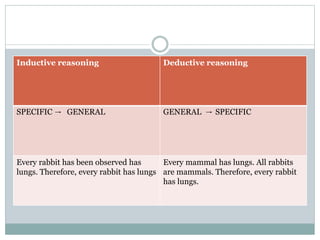
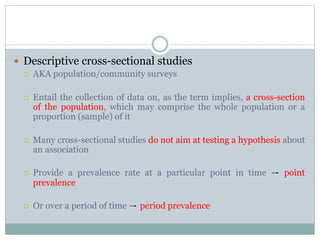
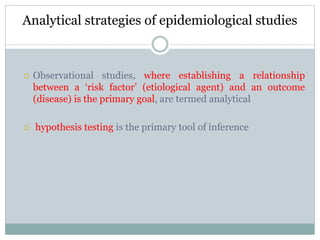
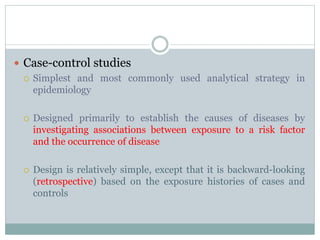
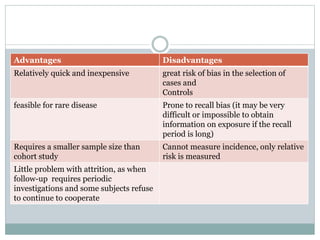
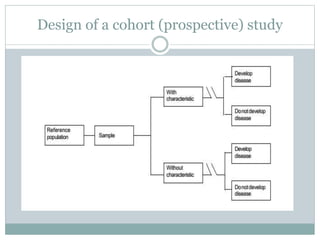
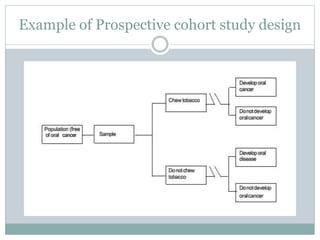
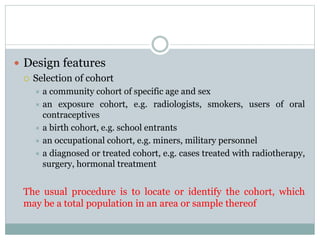
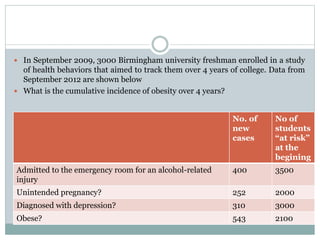
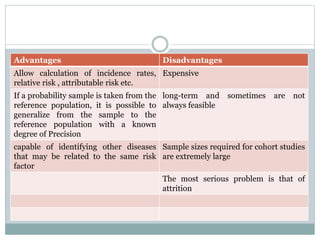
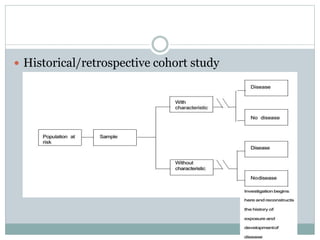
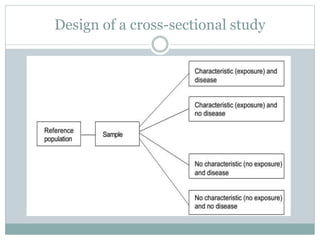
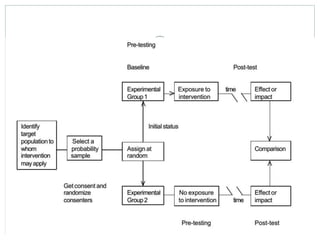
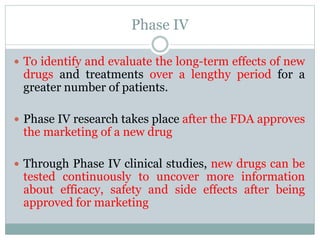
This document discusses health systems research and the research process. It defines research as seeking answers to unanswered questions through planned and systematic collection, analysis, and interpretation of data. The objectives of research can be theoretical, factual, or aimed at application. Characteristics of research include using valid methods to gather new knowledge or data and arriving at careful conclusions. Health systems research seeks to generate knowledge to promote population health. The research process involves selecting a problem, formulating hypotheses, developing a study plan, collecting and analyzing data, and formulating results.