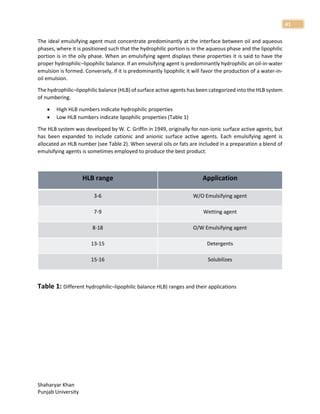
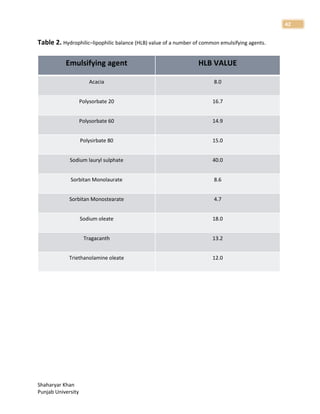
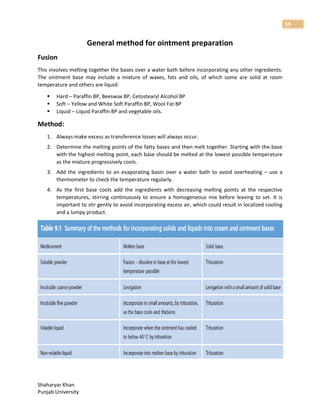
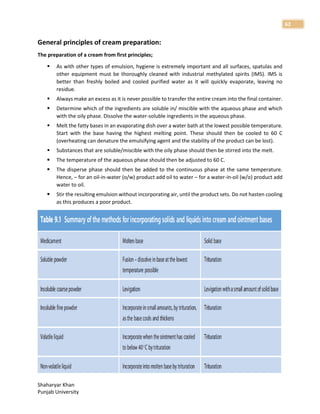
1. The document discusses basic principles of compounding and dispensing including weights and measures, calculations, fundamental operations in compounding, containers and closures for dispensed products, and prescription handling.
2. It defines key terms like compounding, prescription, and describes the parts of a prescription including prescriber information, patient information, inscription, subscription, and refill information.
3. Guidelines are provided for different dosage forms including liquids, semi-solids, solids, and powders. Appropriate containers and closures are discussed for each type.
4. The document also covers prescription handling, labeling requirements, and fundamental operations involved in compounding like weights and measures, size reduction,