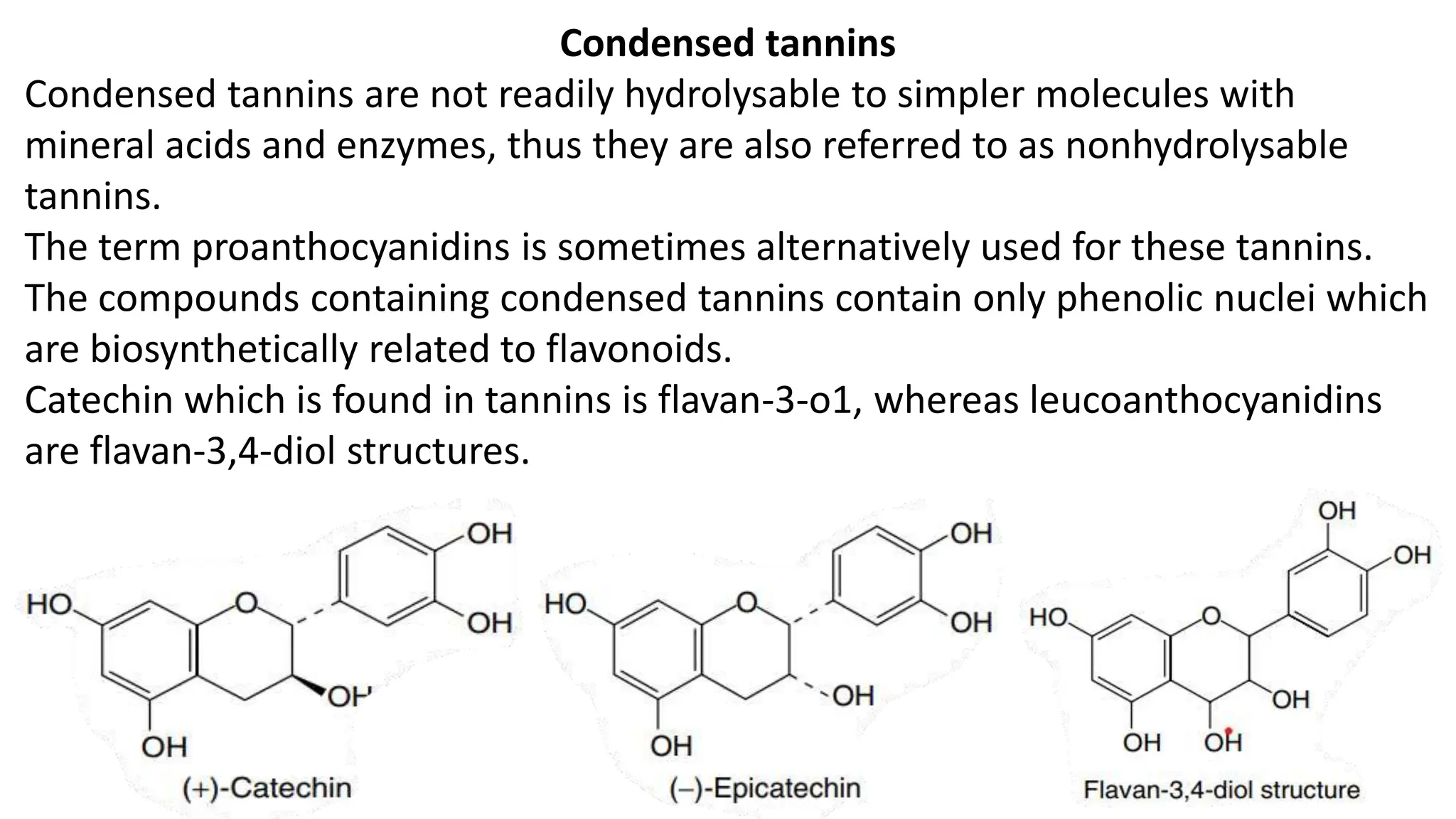
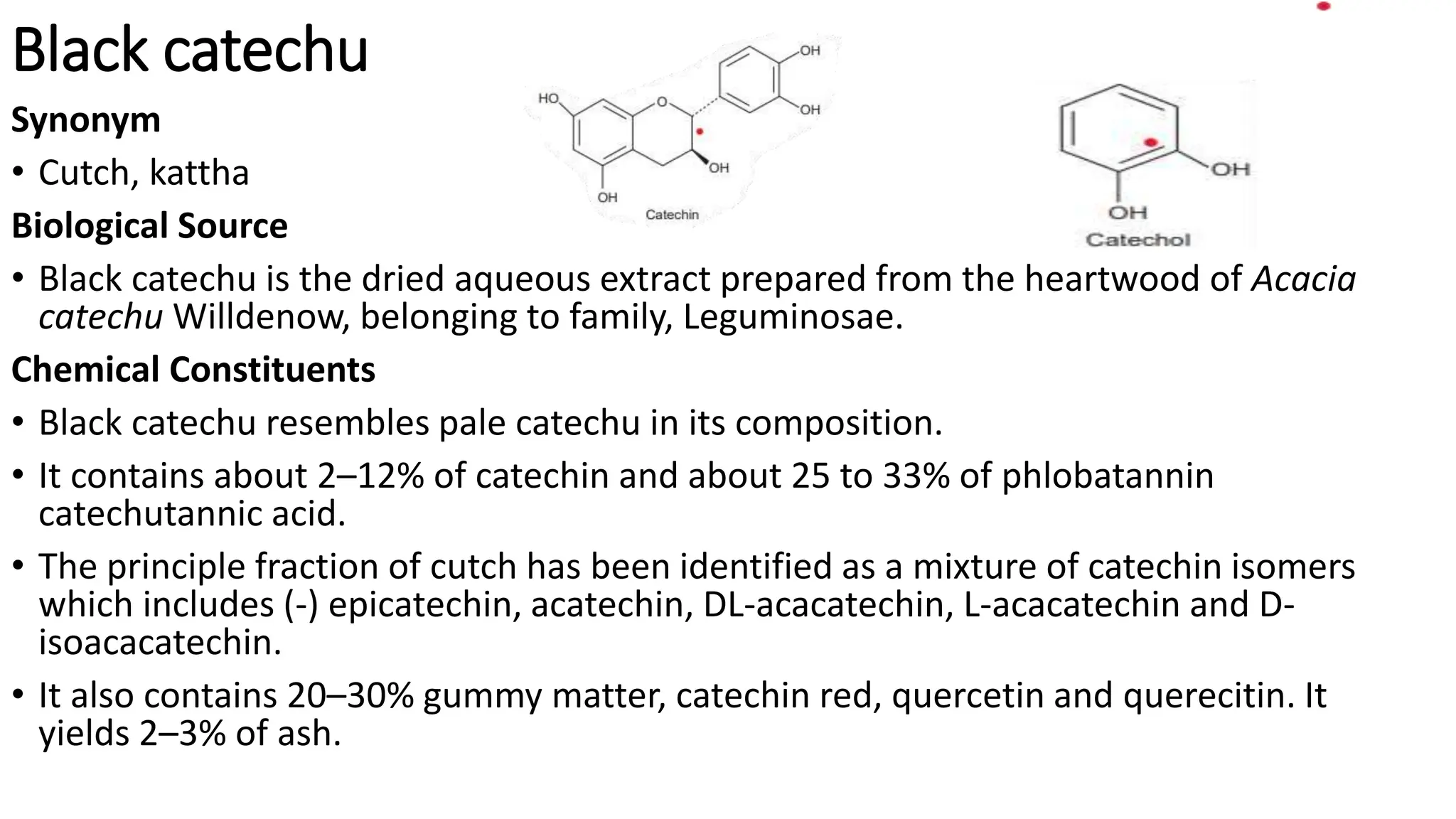
Tannins are complex organic polyphenols derived from plants that exhibit astringent properties and are classified into true tannins and pseudotannins based on their ability to bind with proteins. They can be further categorized into hydrolysable and condensed tannins, with hydrolysable tannins being soluble in water and derived from phenolic acids, while condensed tannins are non-hydrolysable and linked to flavonoids. Tannins have various applications in the leather industry, herbal medicine, food and beverage taste, and nutritional value for herbivores.