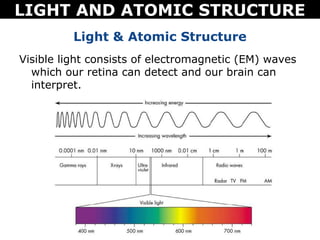
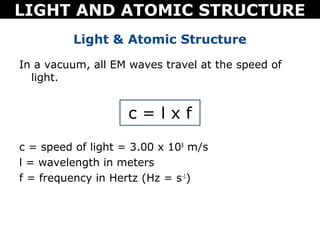
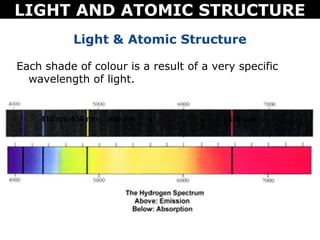
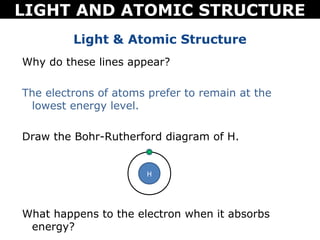
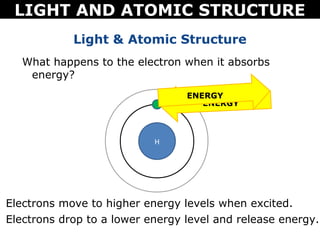
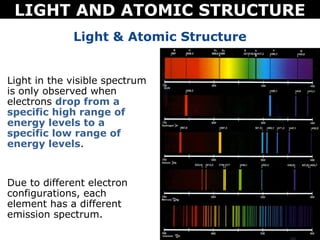
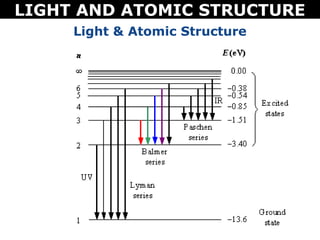
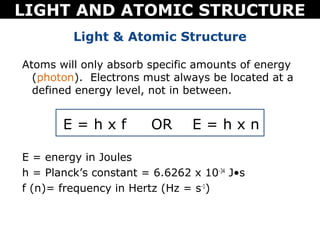
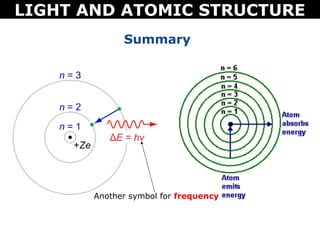
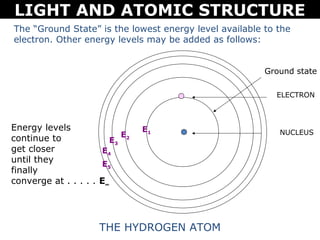
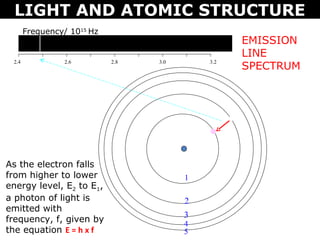
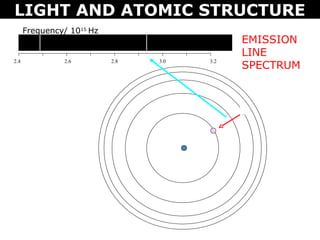
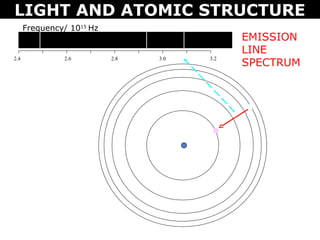
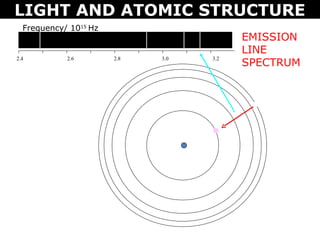
The document discusses light and atomic structure. It explains that electrons can only occupy certain energy levels within an atom and will absorb or emit photons of light of specific wavelengths as they move between these levels. The document also introduces the Bohr model of the hydrogen atom and how electrons transitioning between energy levels leads to emission spectra that are unique for each element.