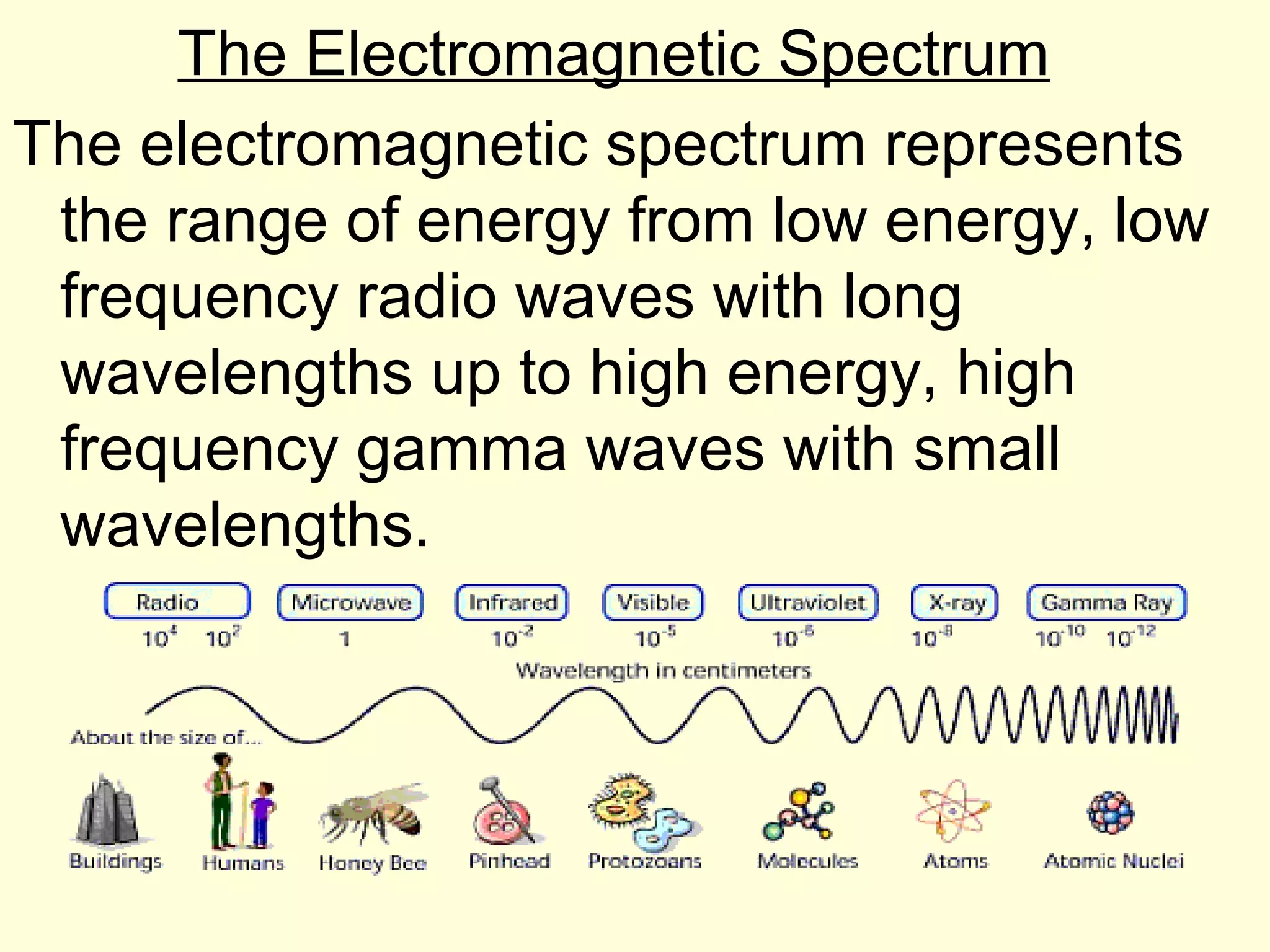
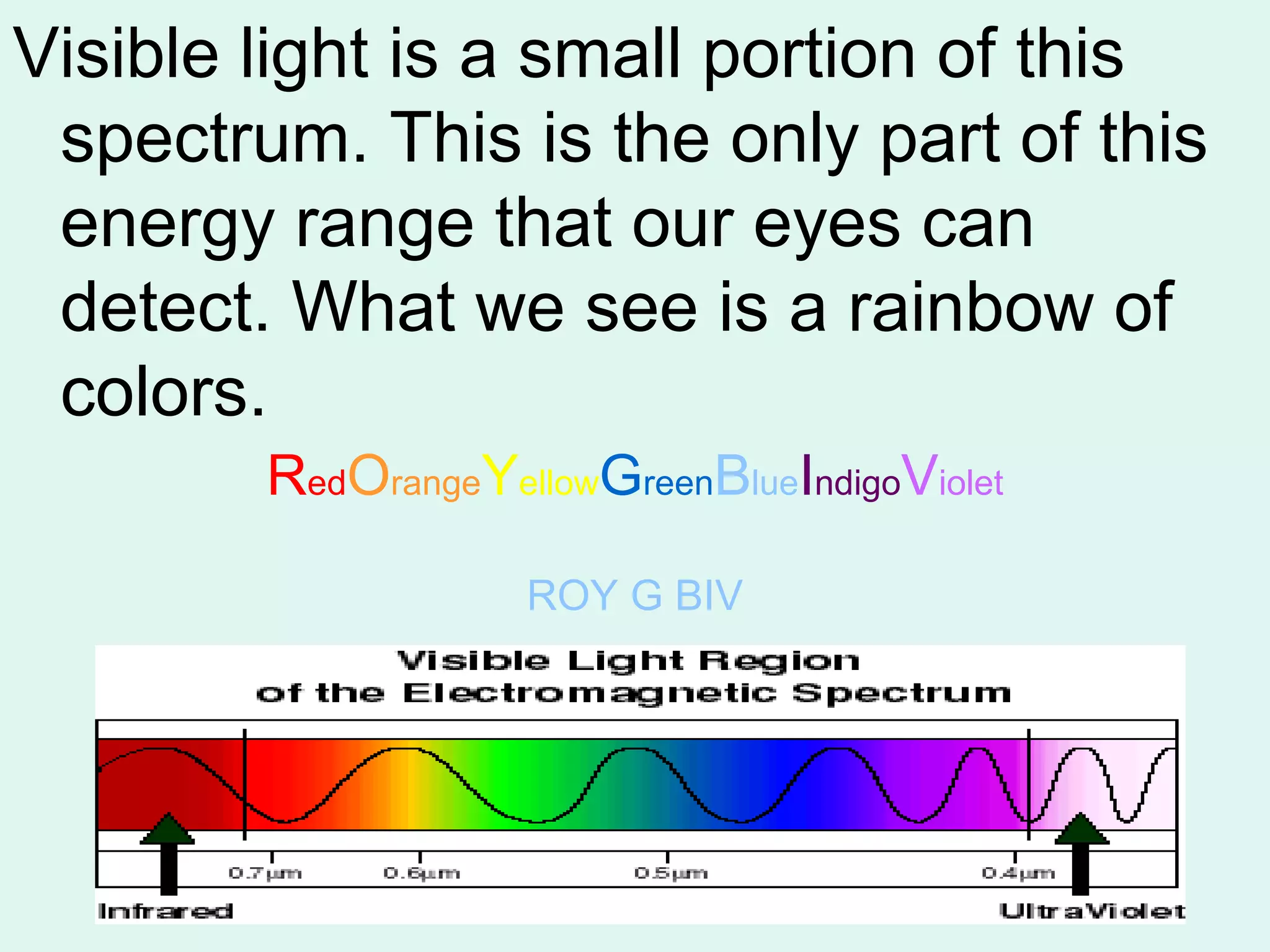
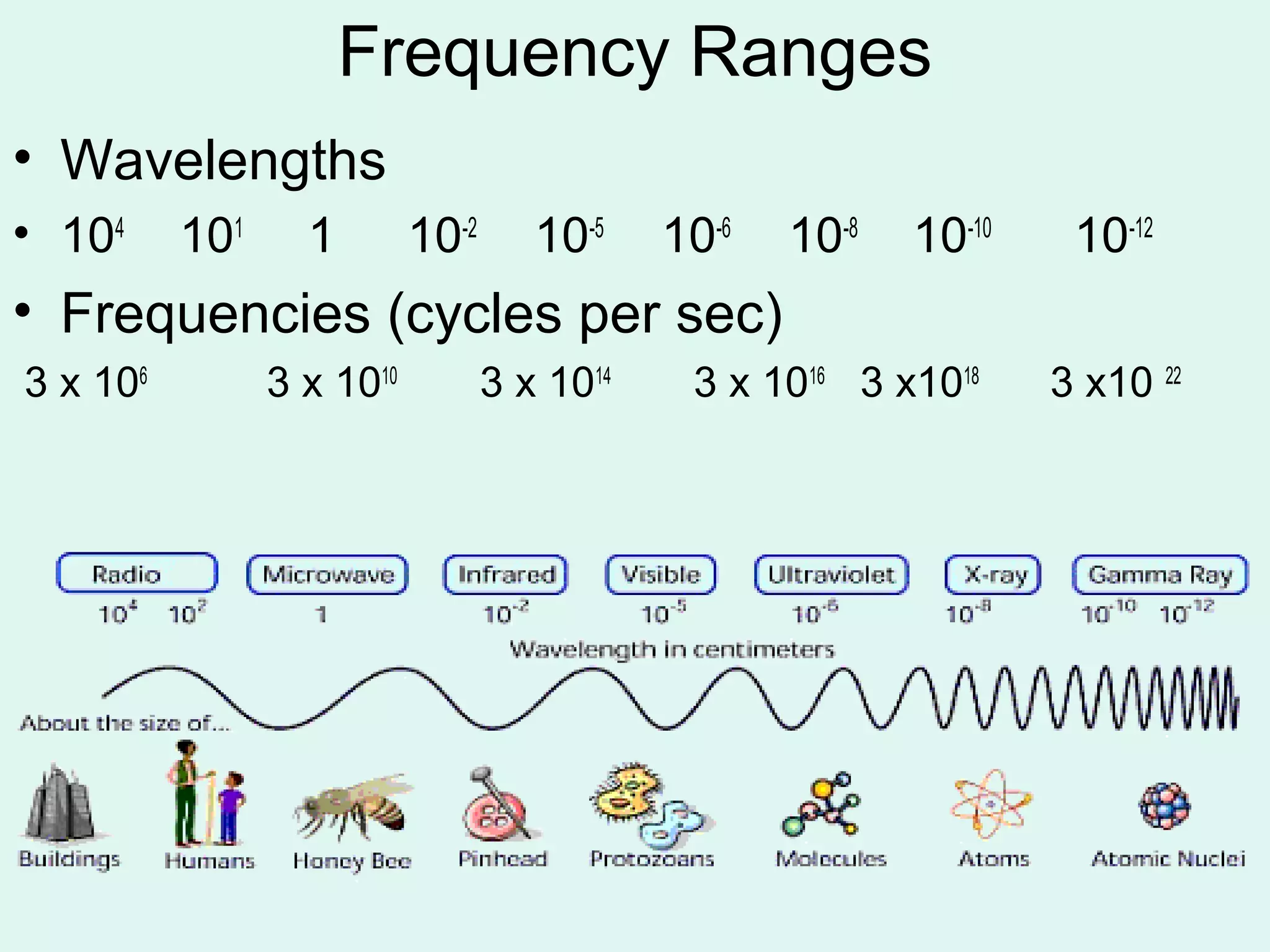
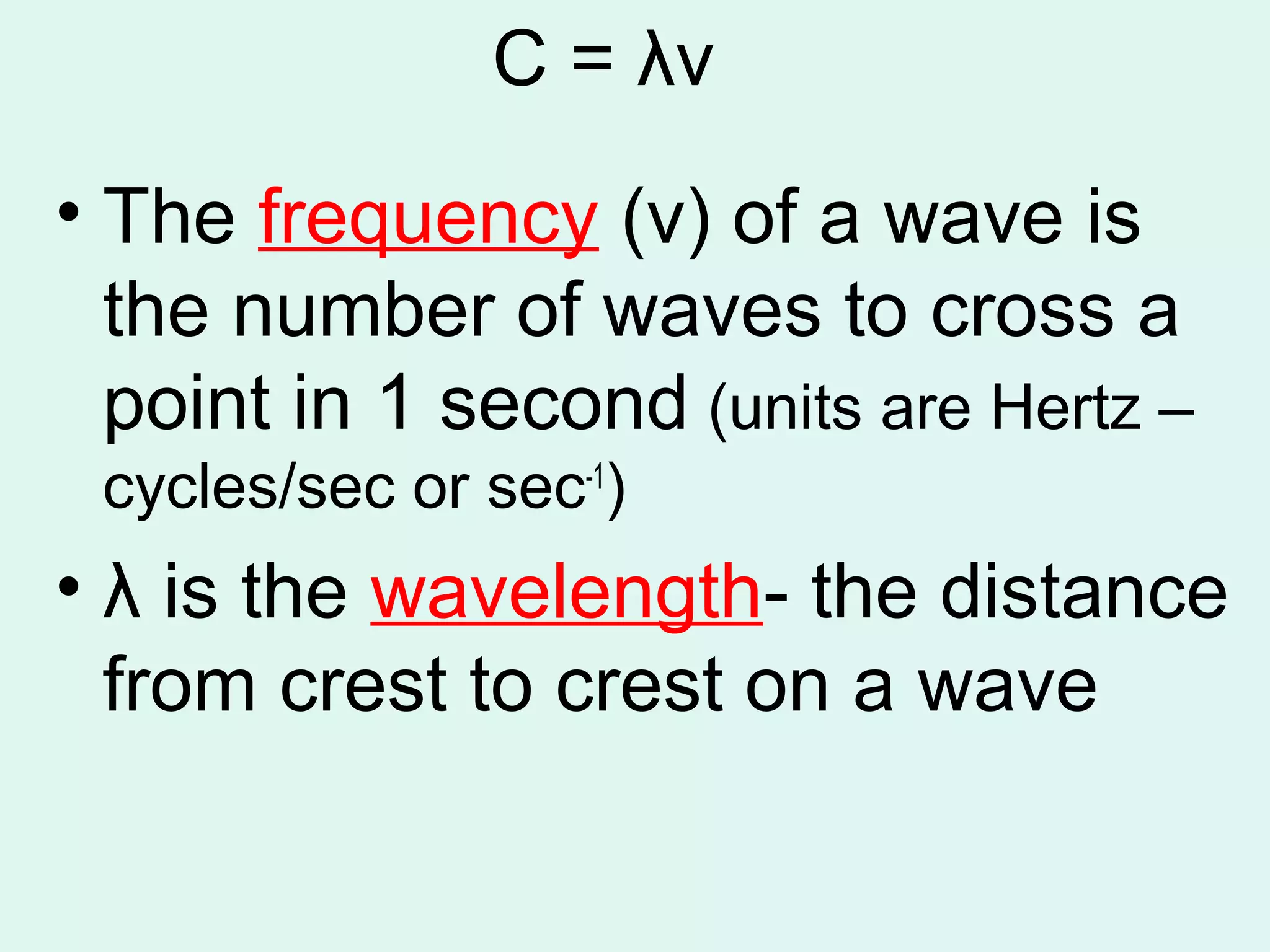
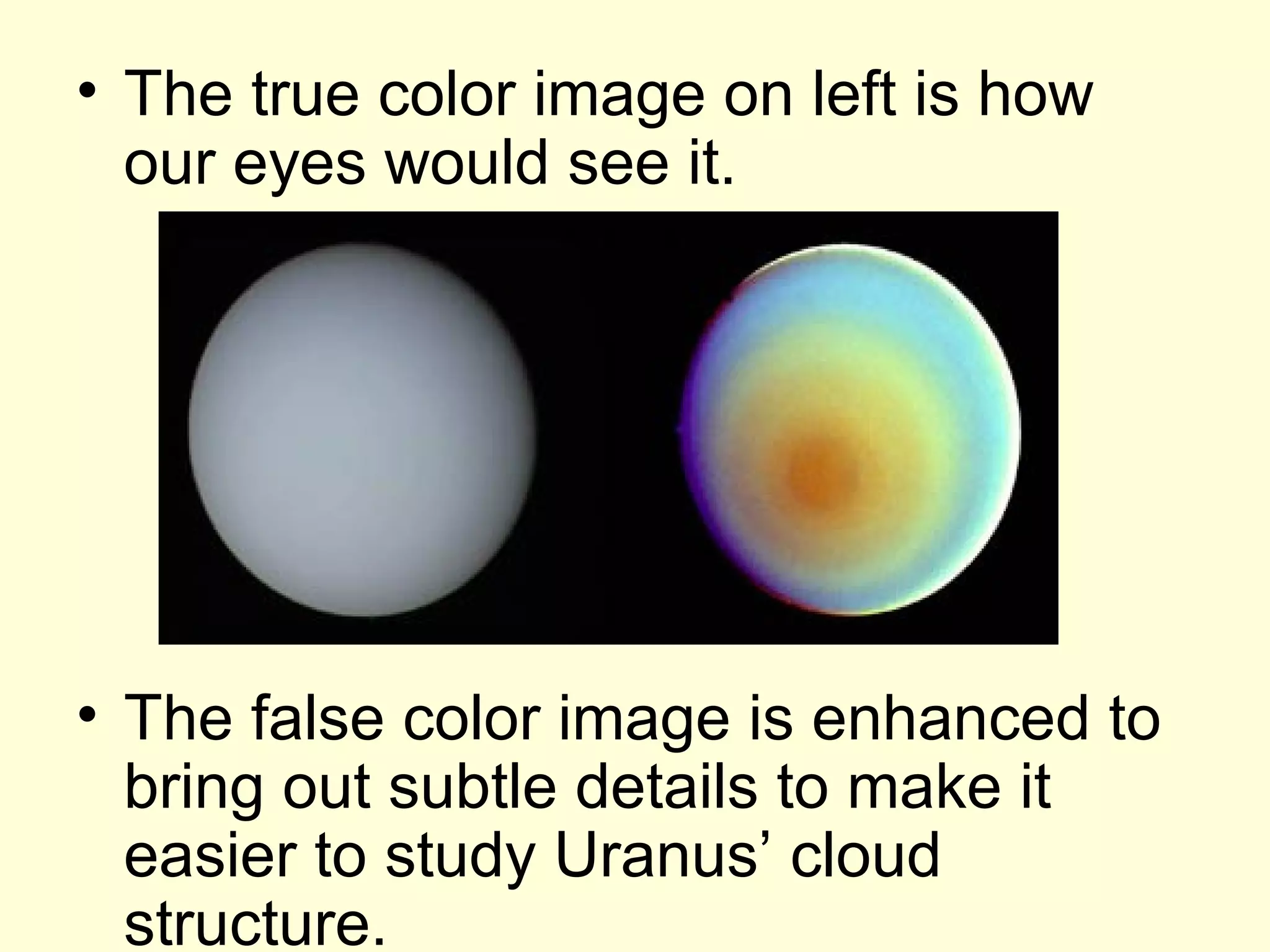
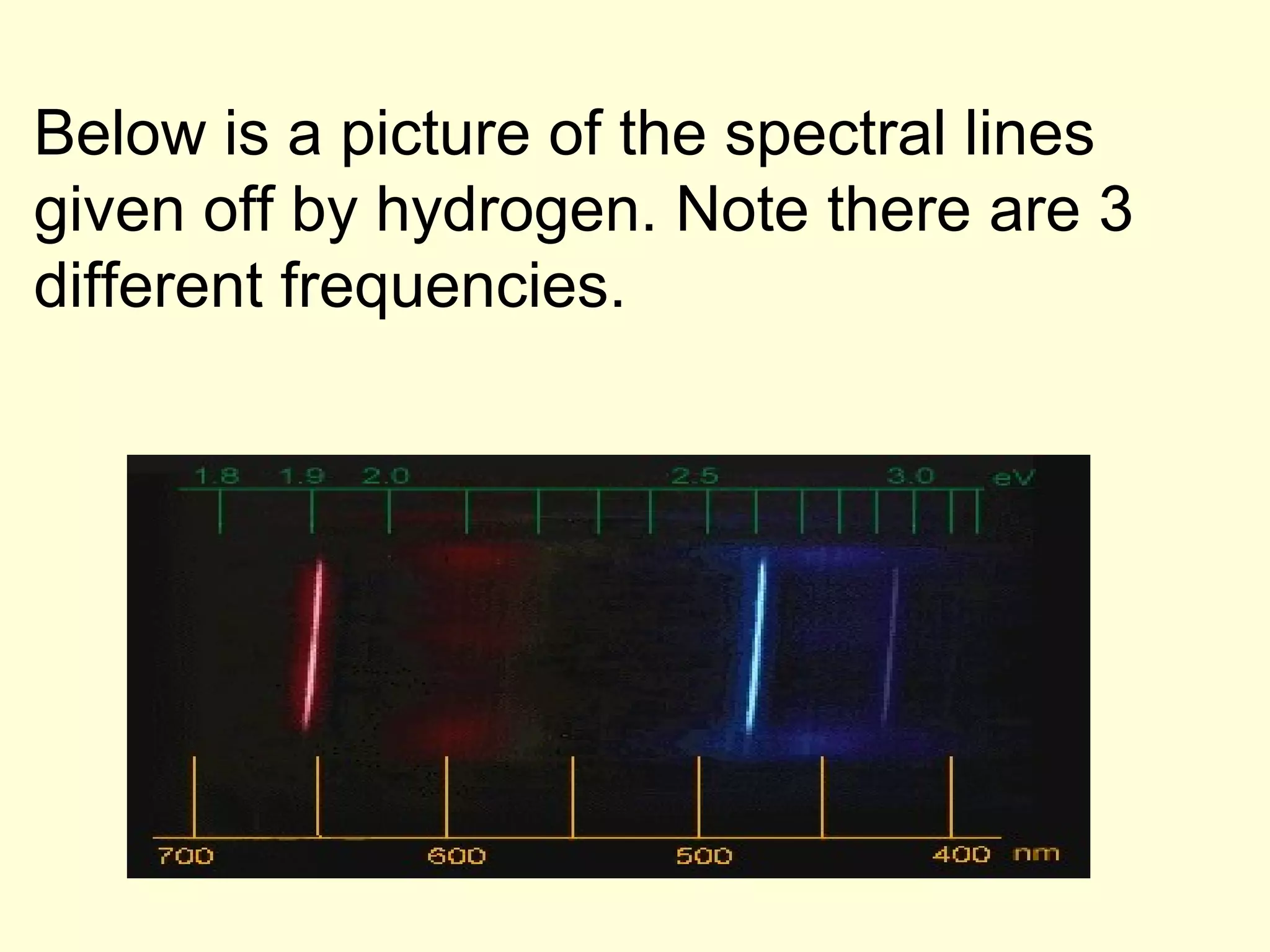
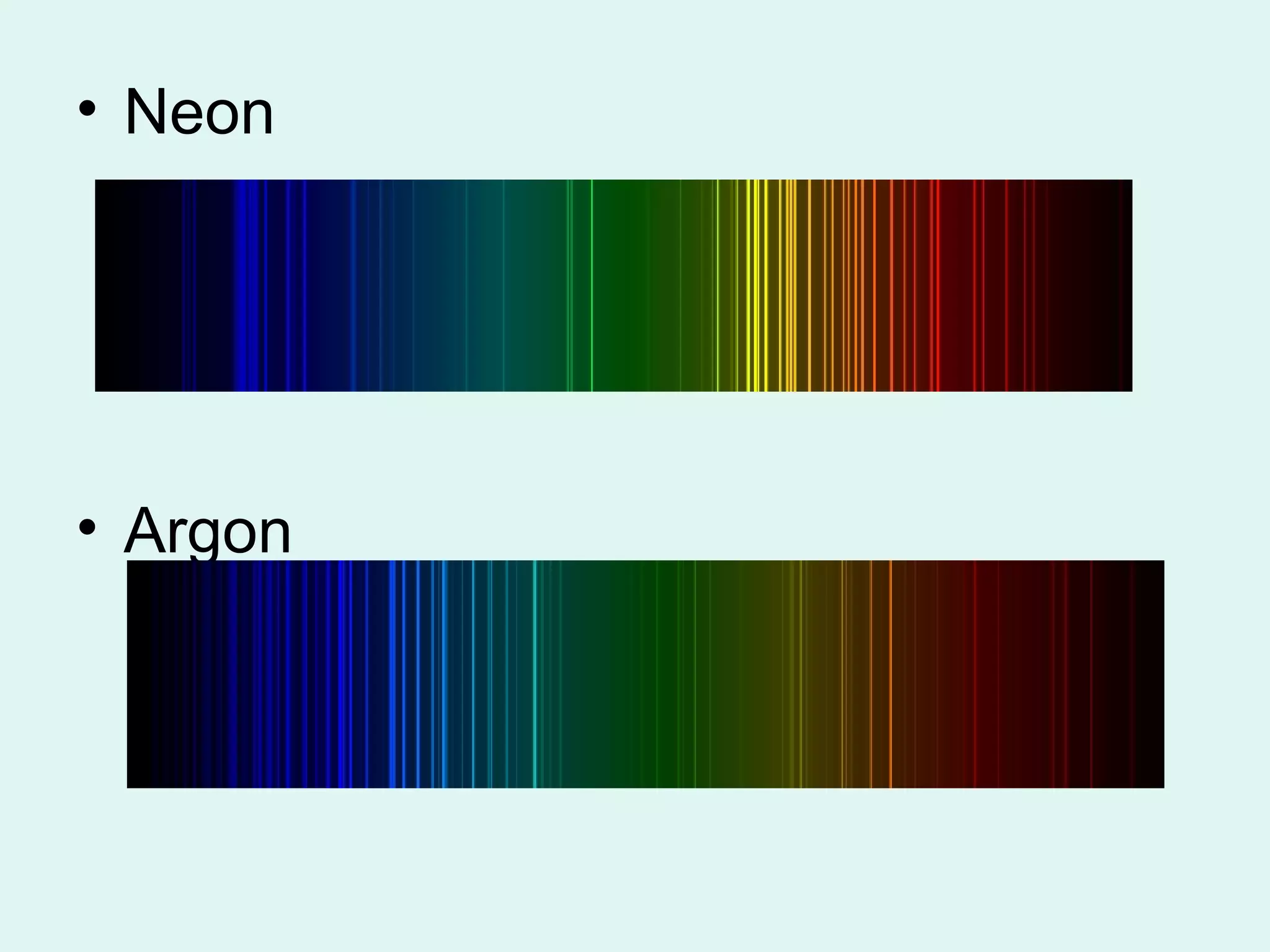
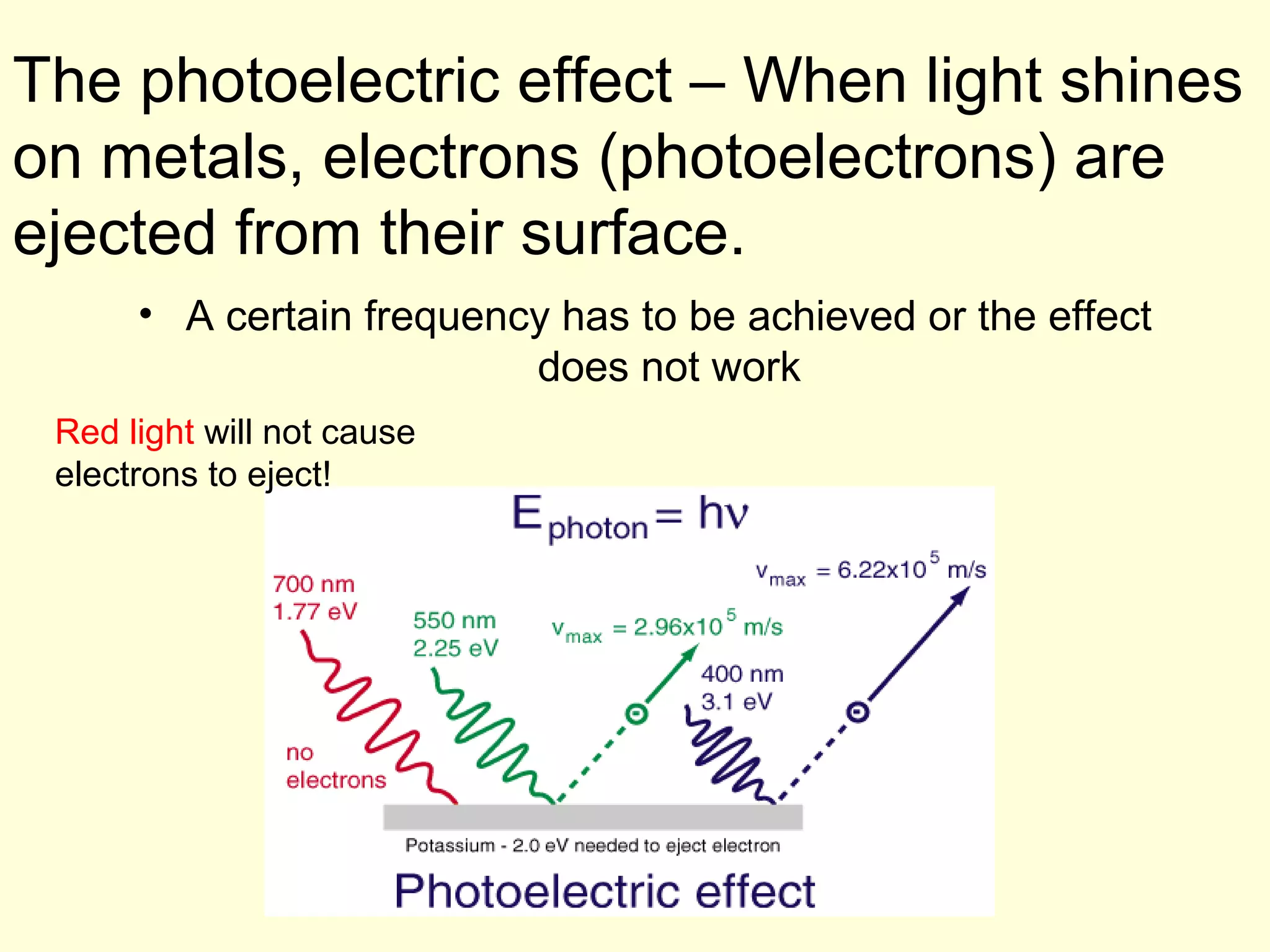
The document provides information about light and the electromagnetic spectrum. It discusses how light can behave as both a particle and wave, and defines the electromagnetic spectrum as ranging from low frequency radio waves to high frequency gamma rays. It notes that visible light is a small portion of the spectrum detectable by human eyes.