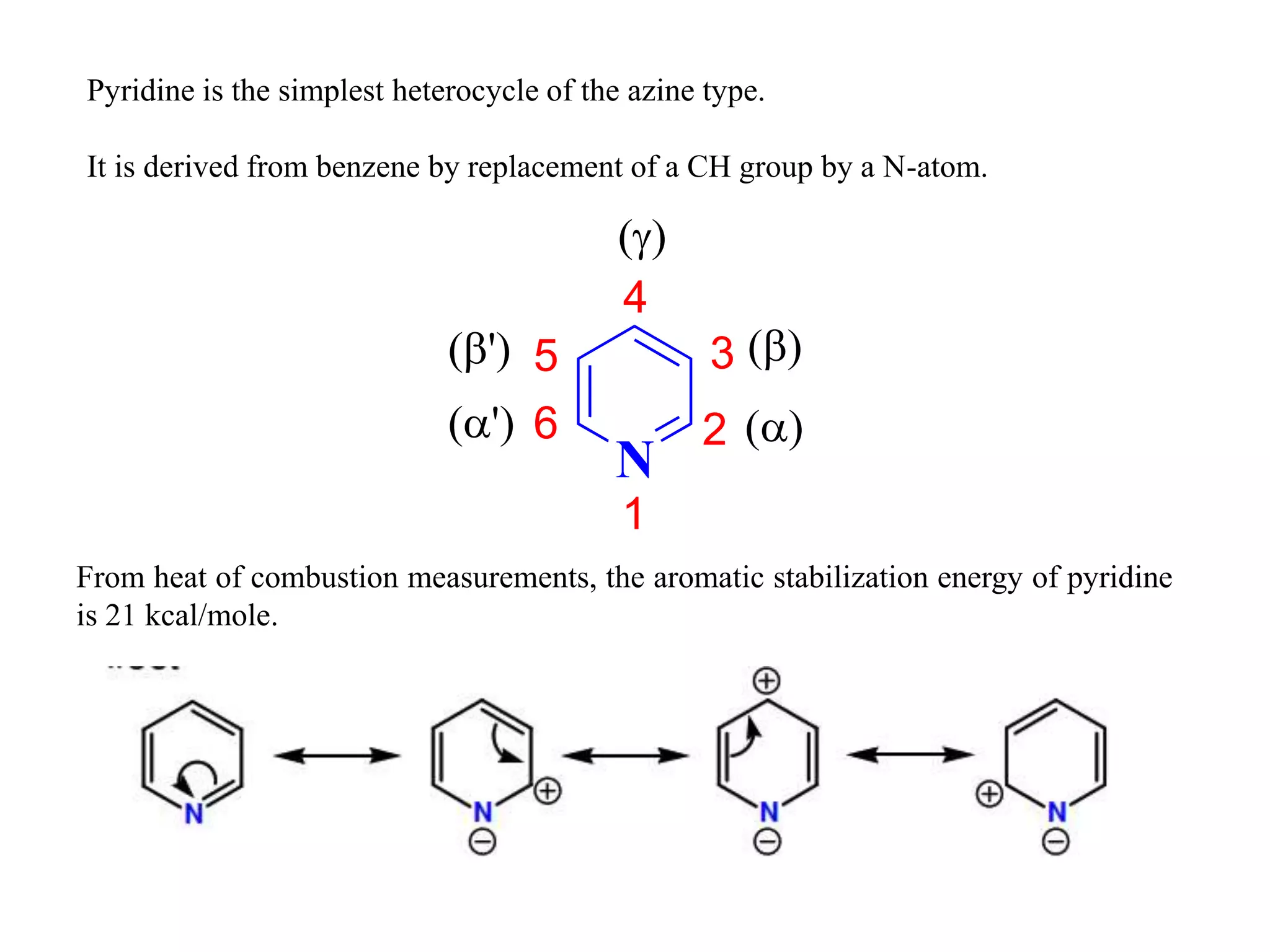
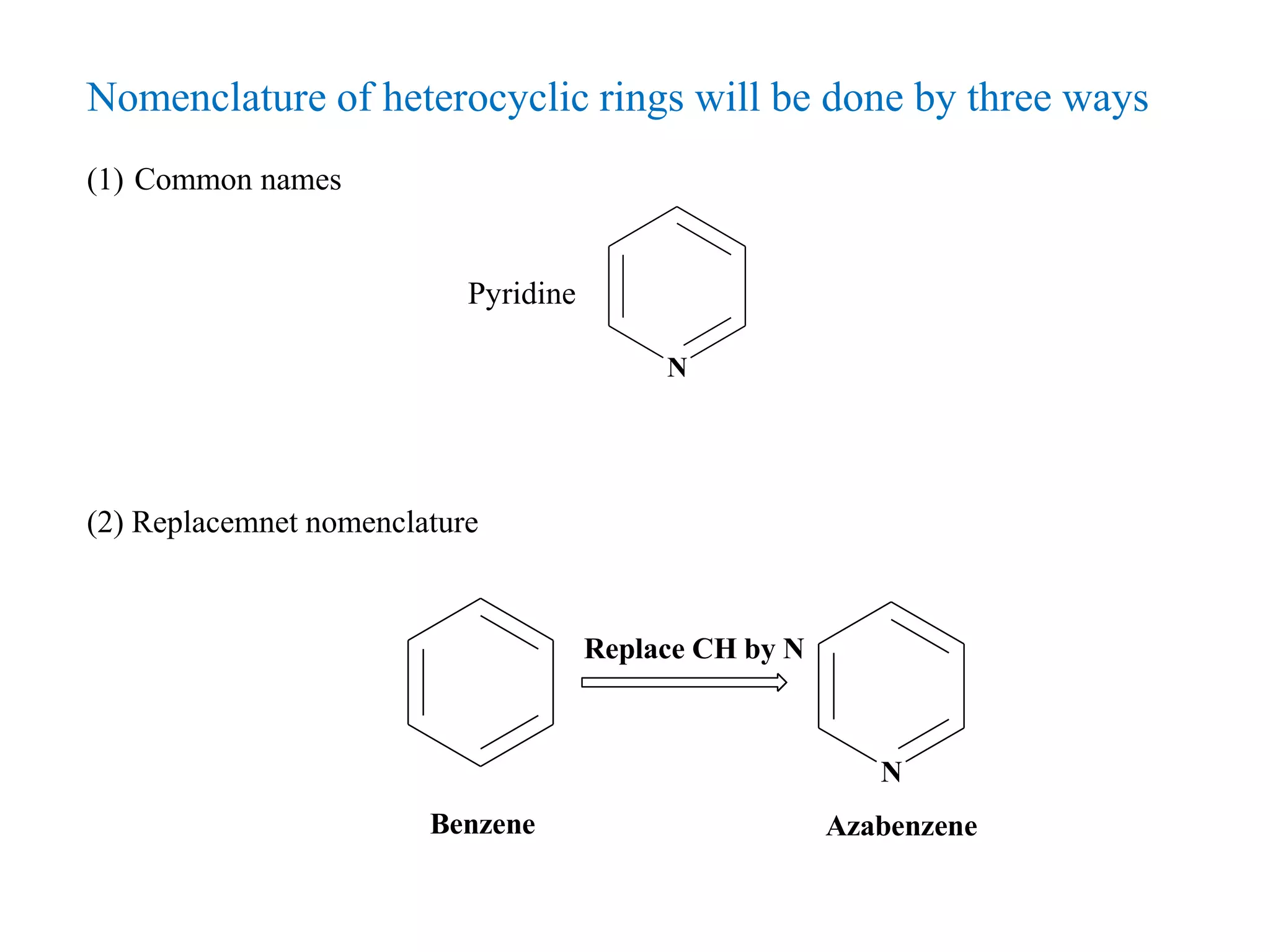
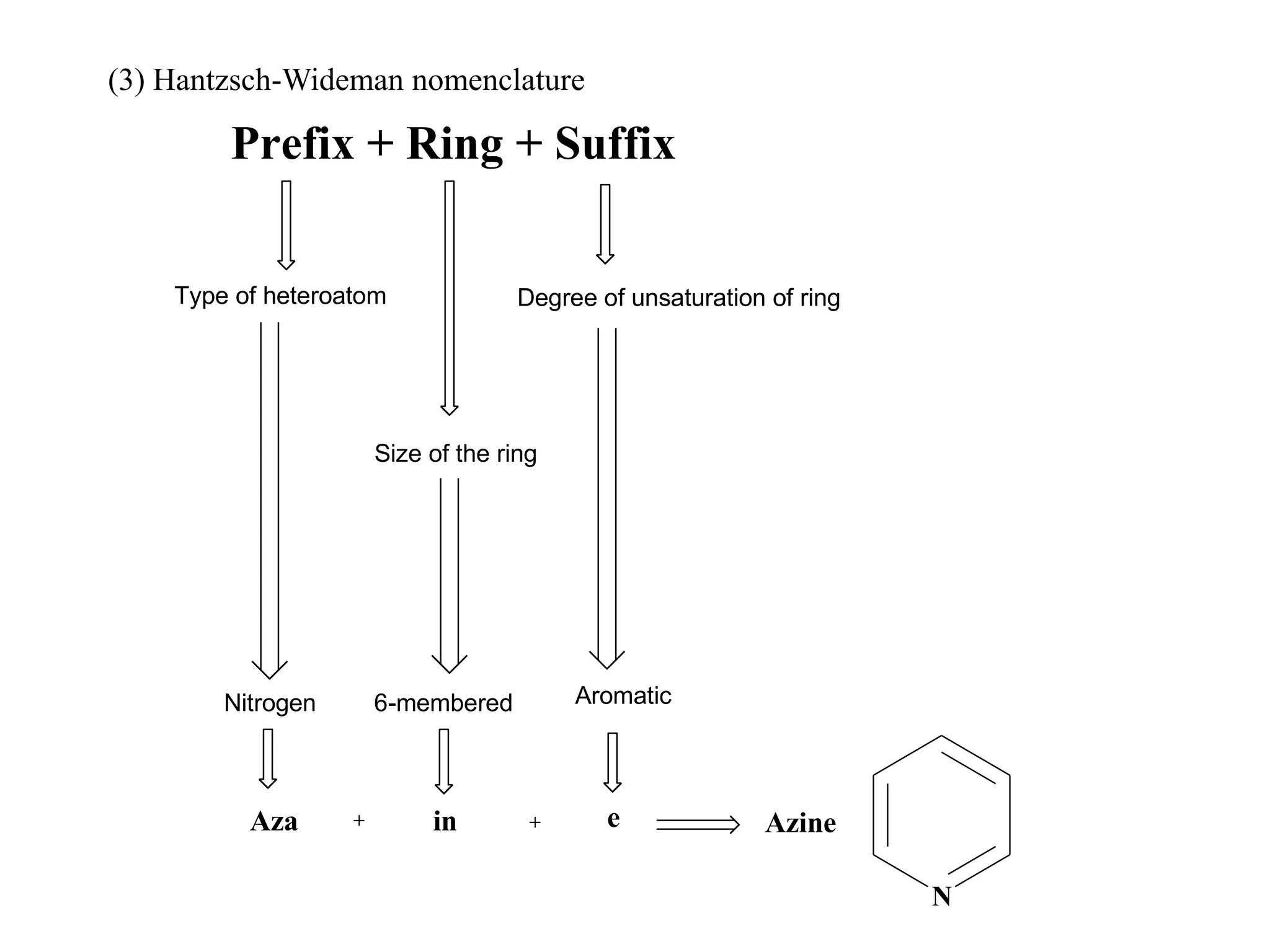
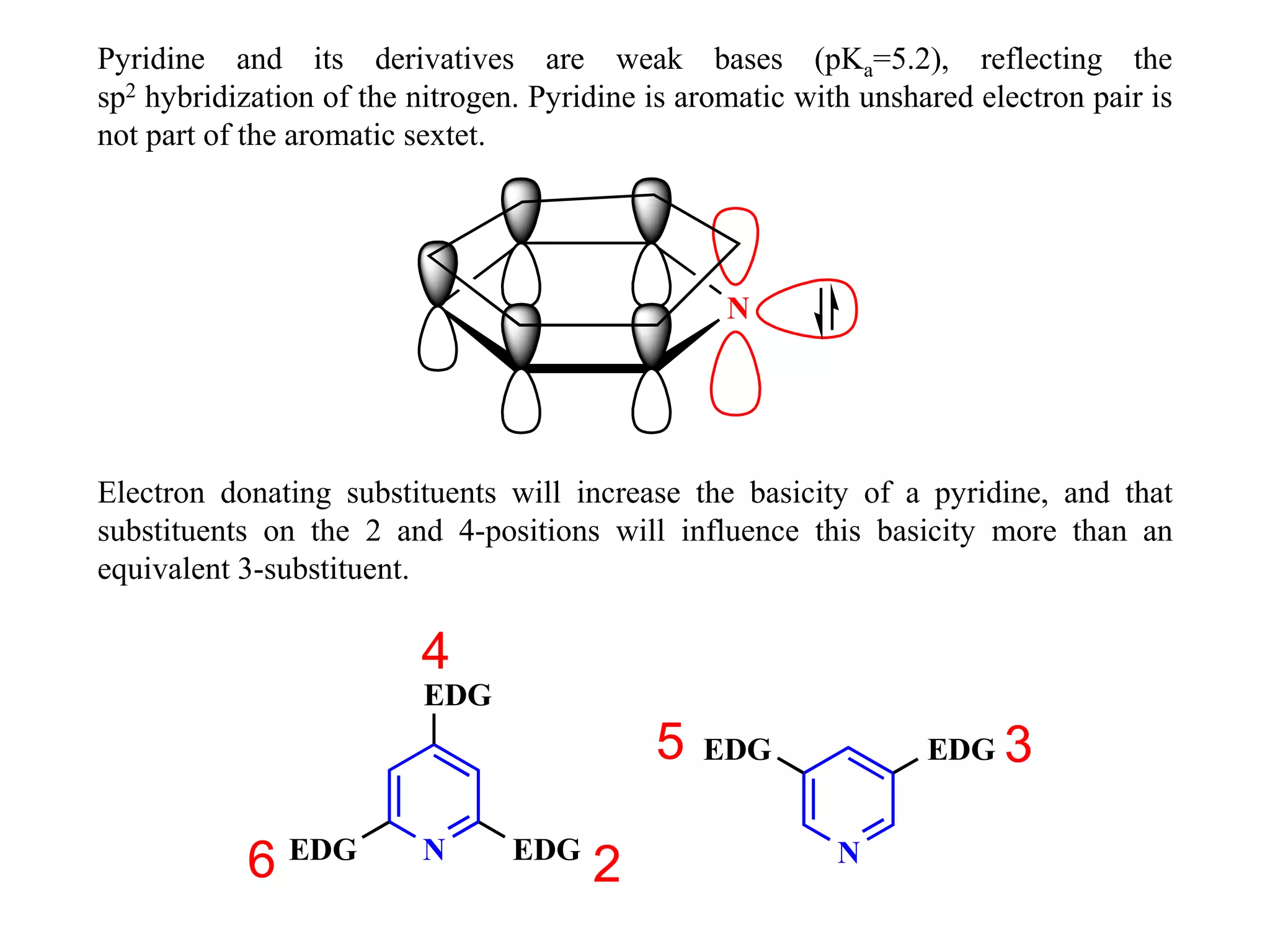
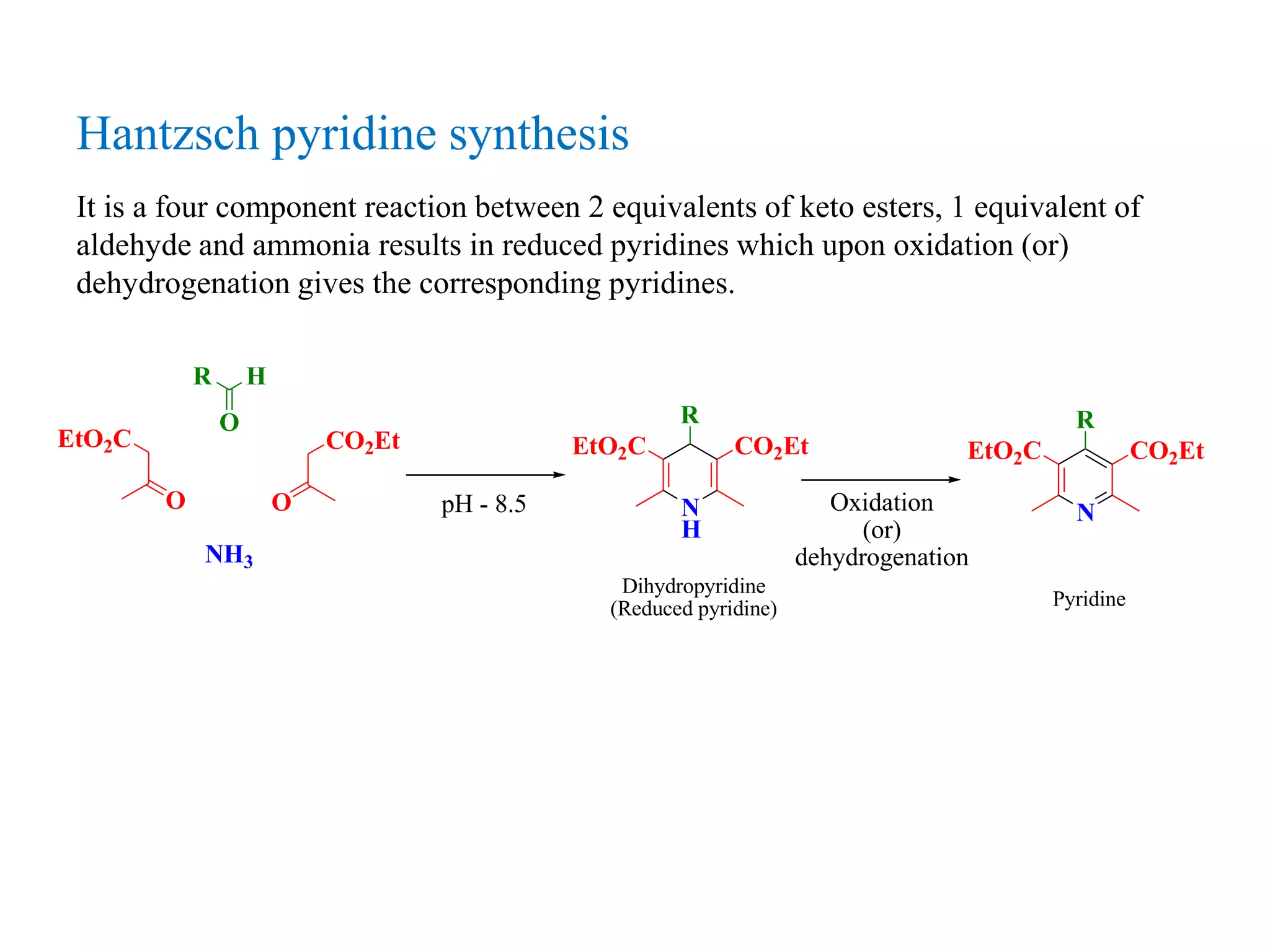
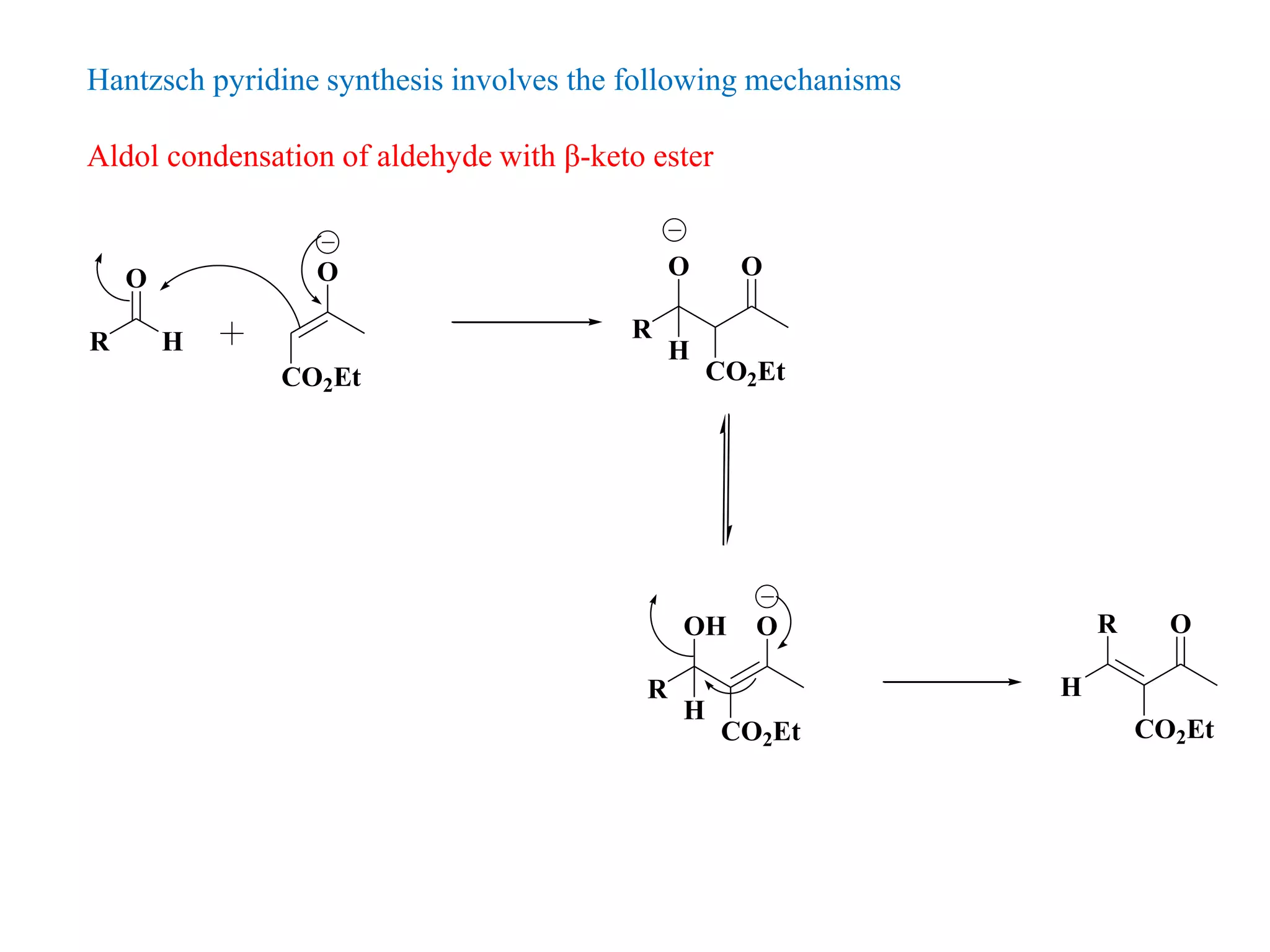
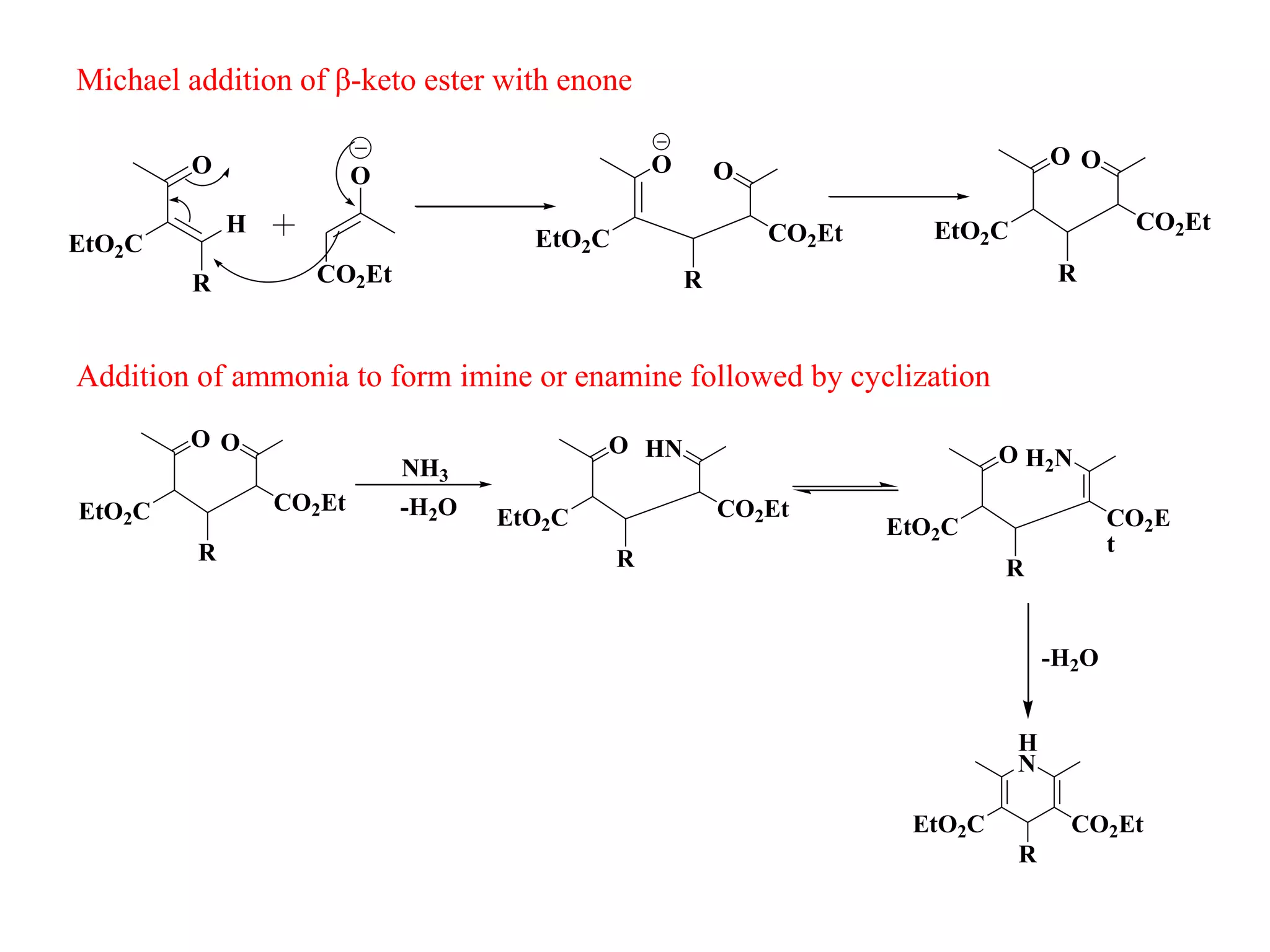
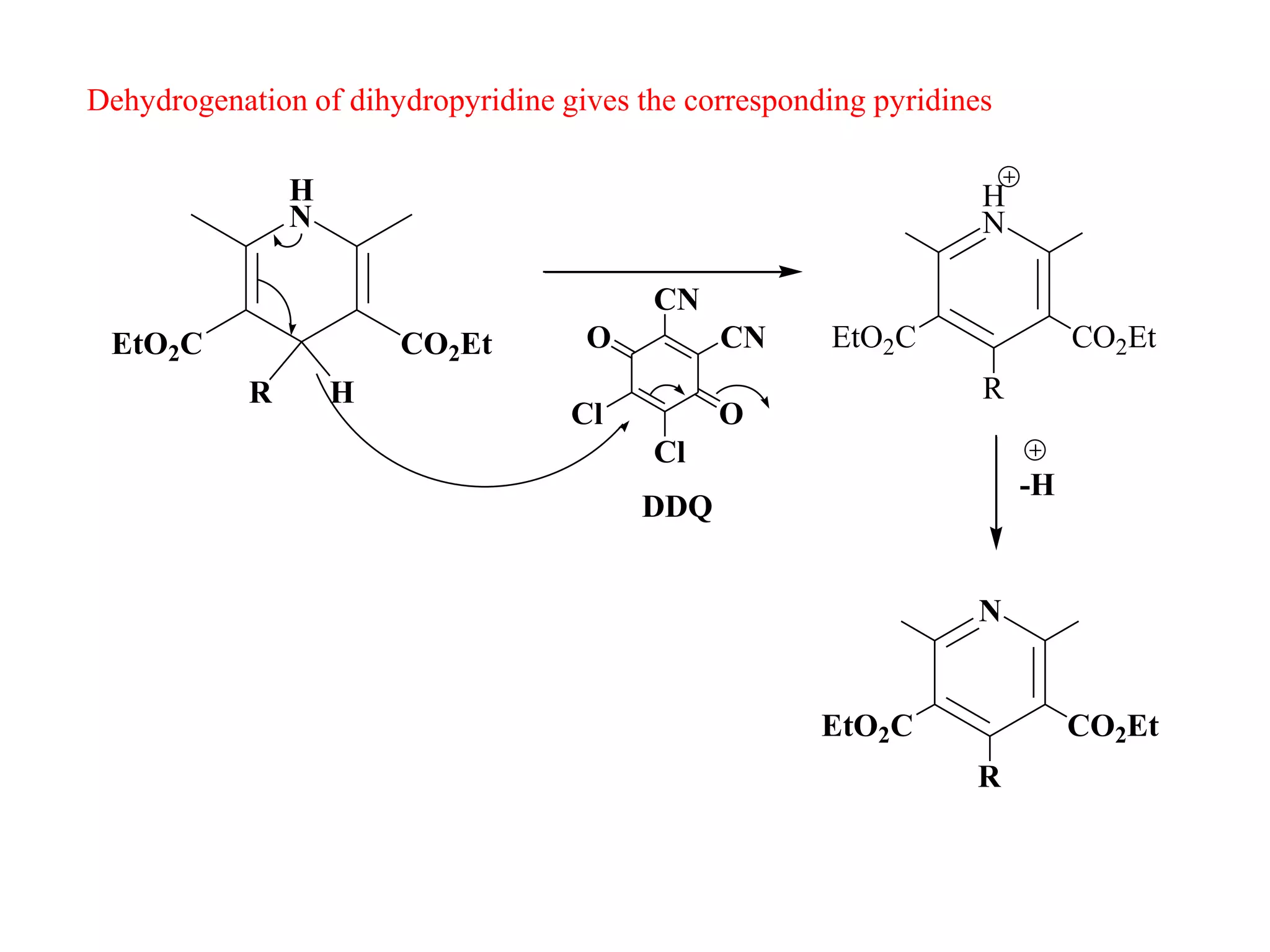
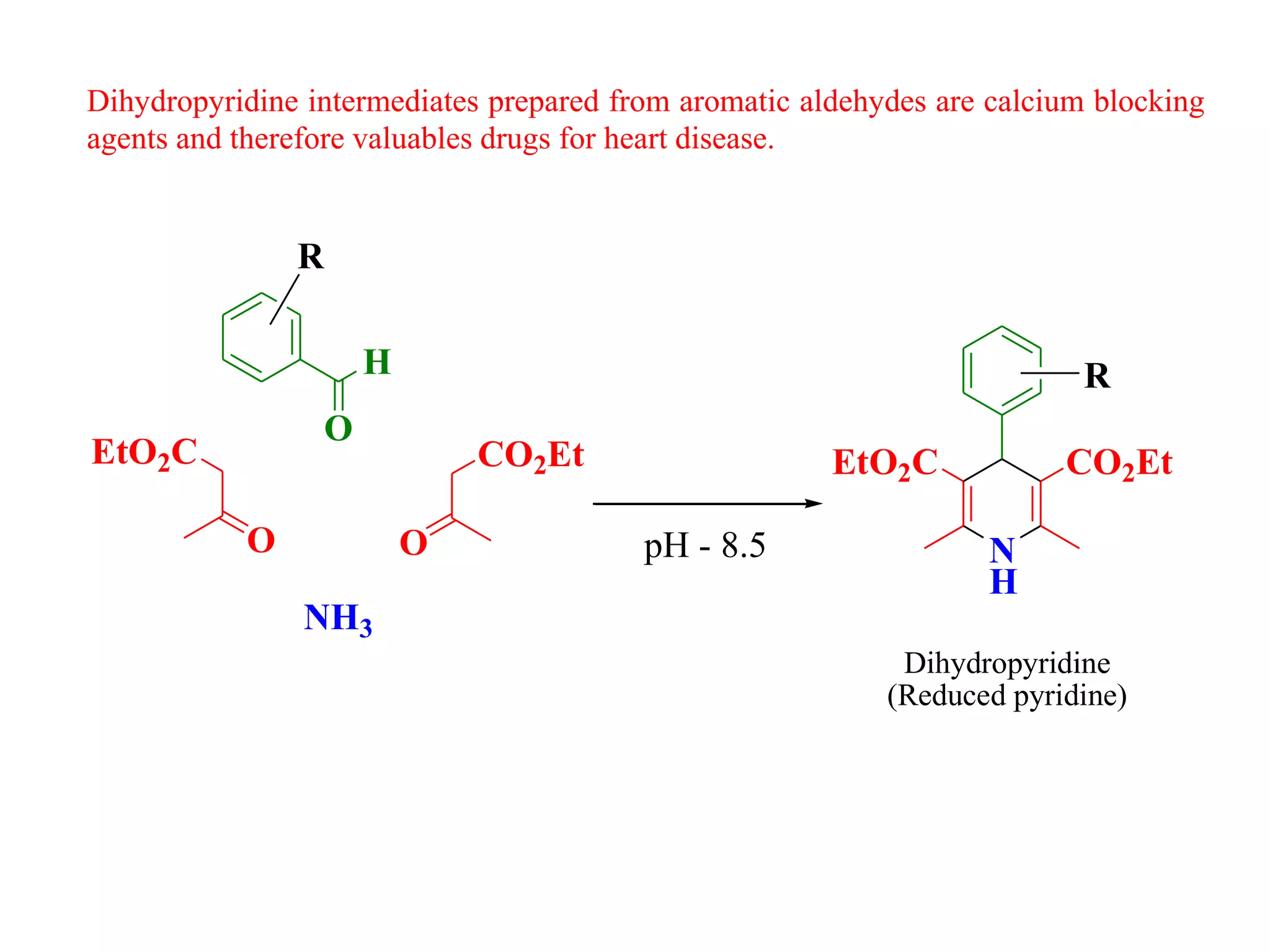
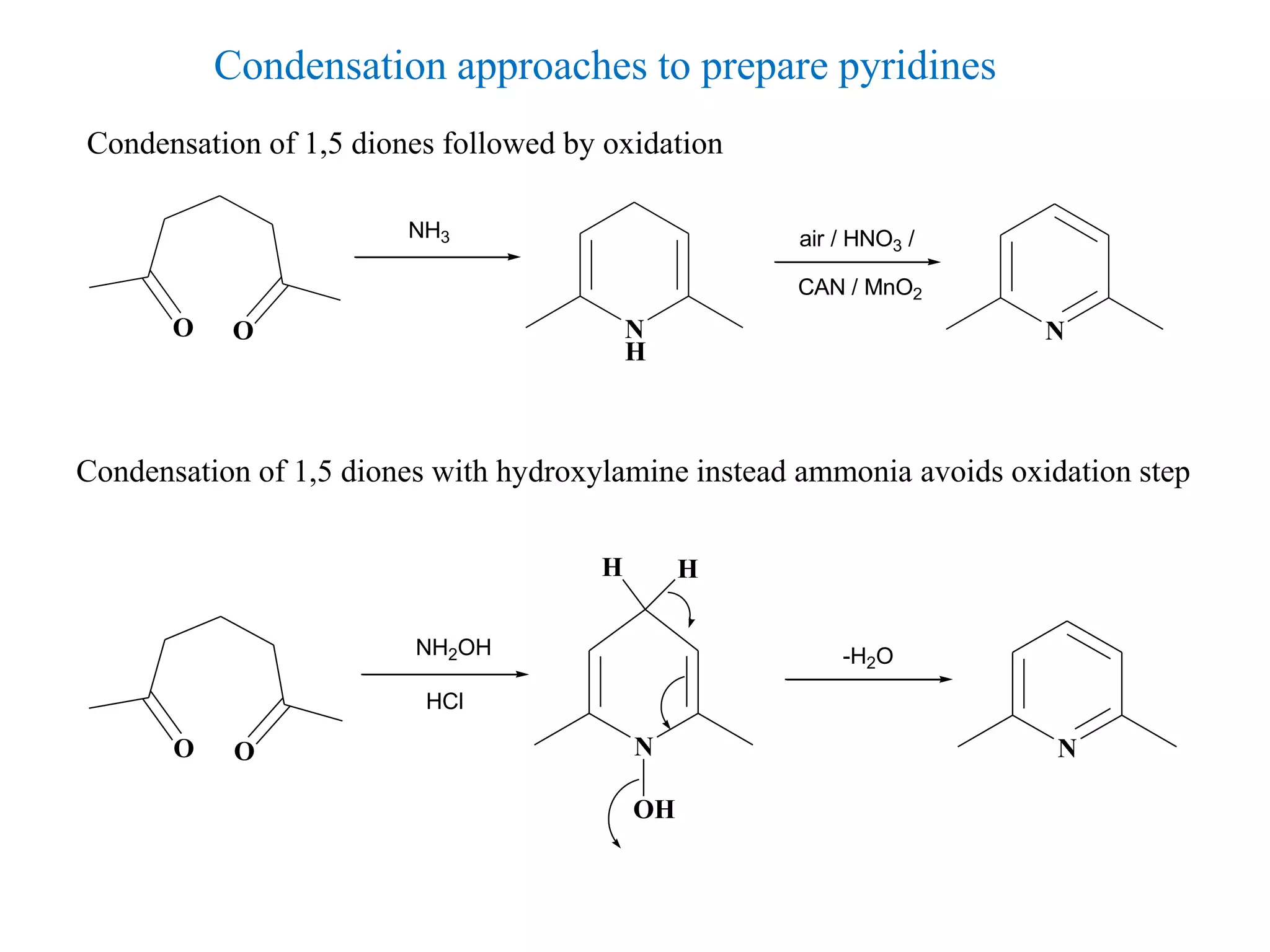
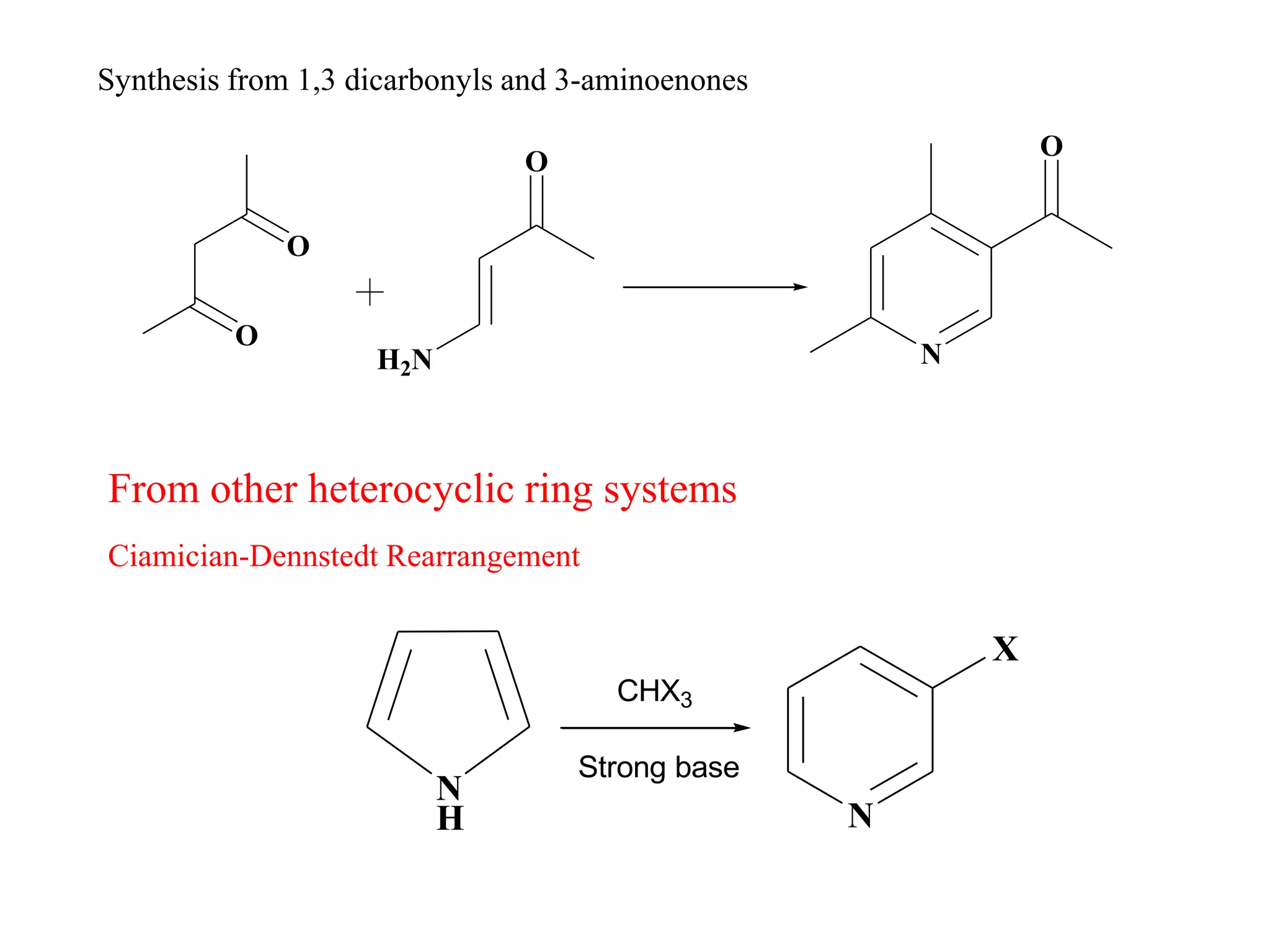
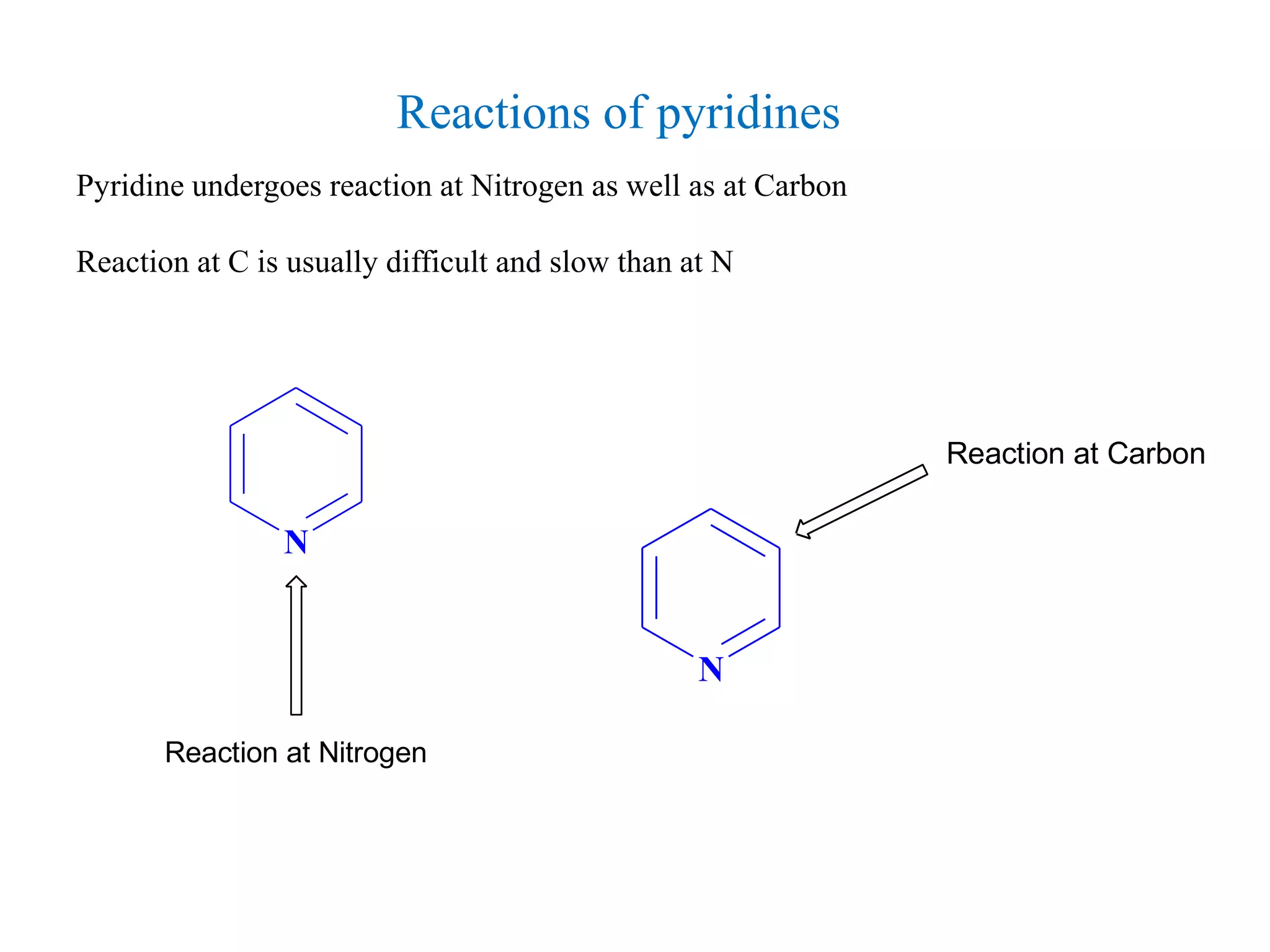
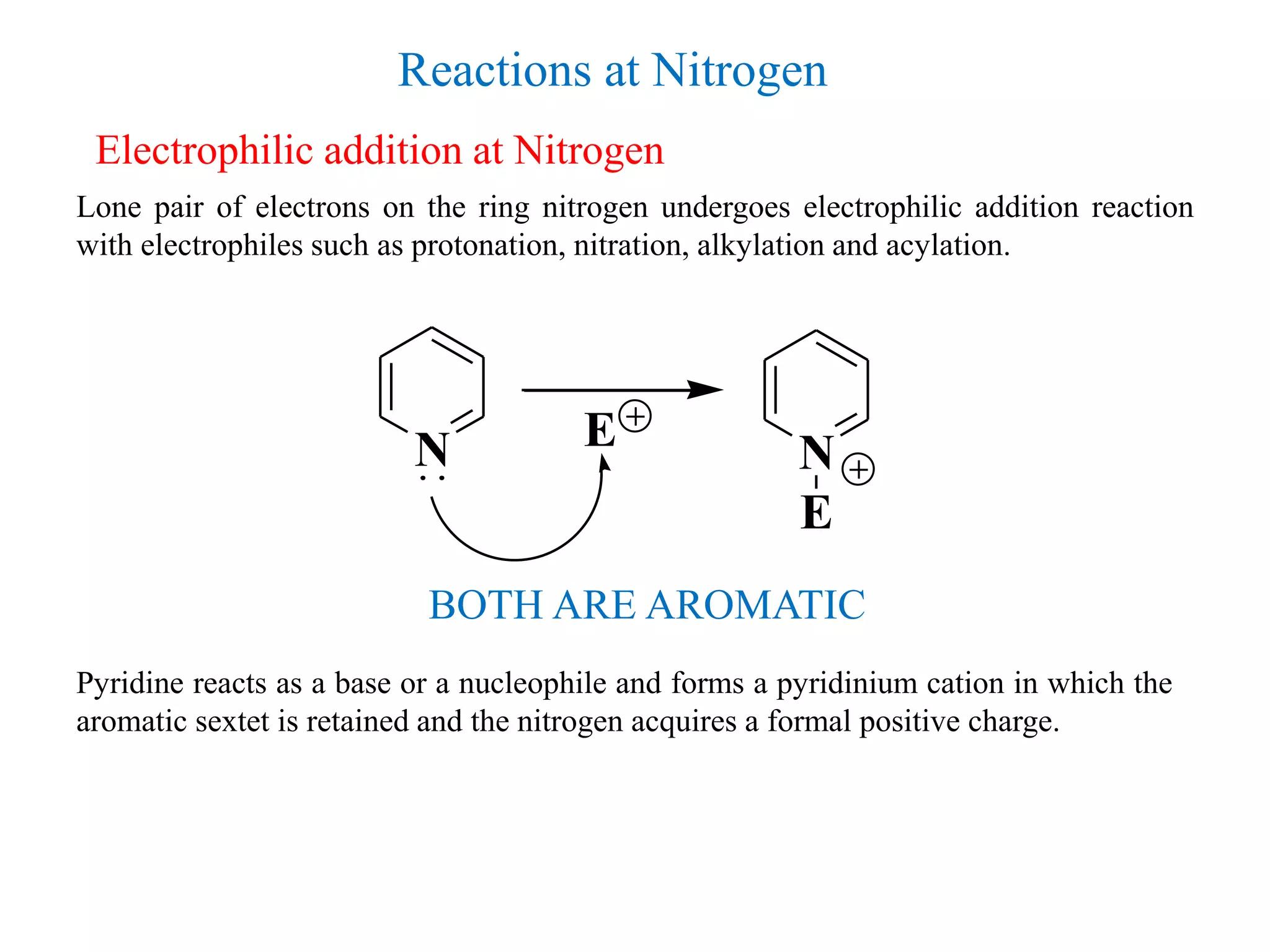
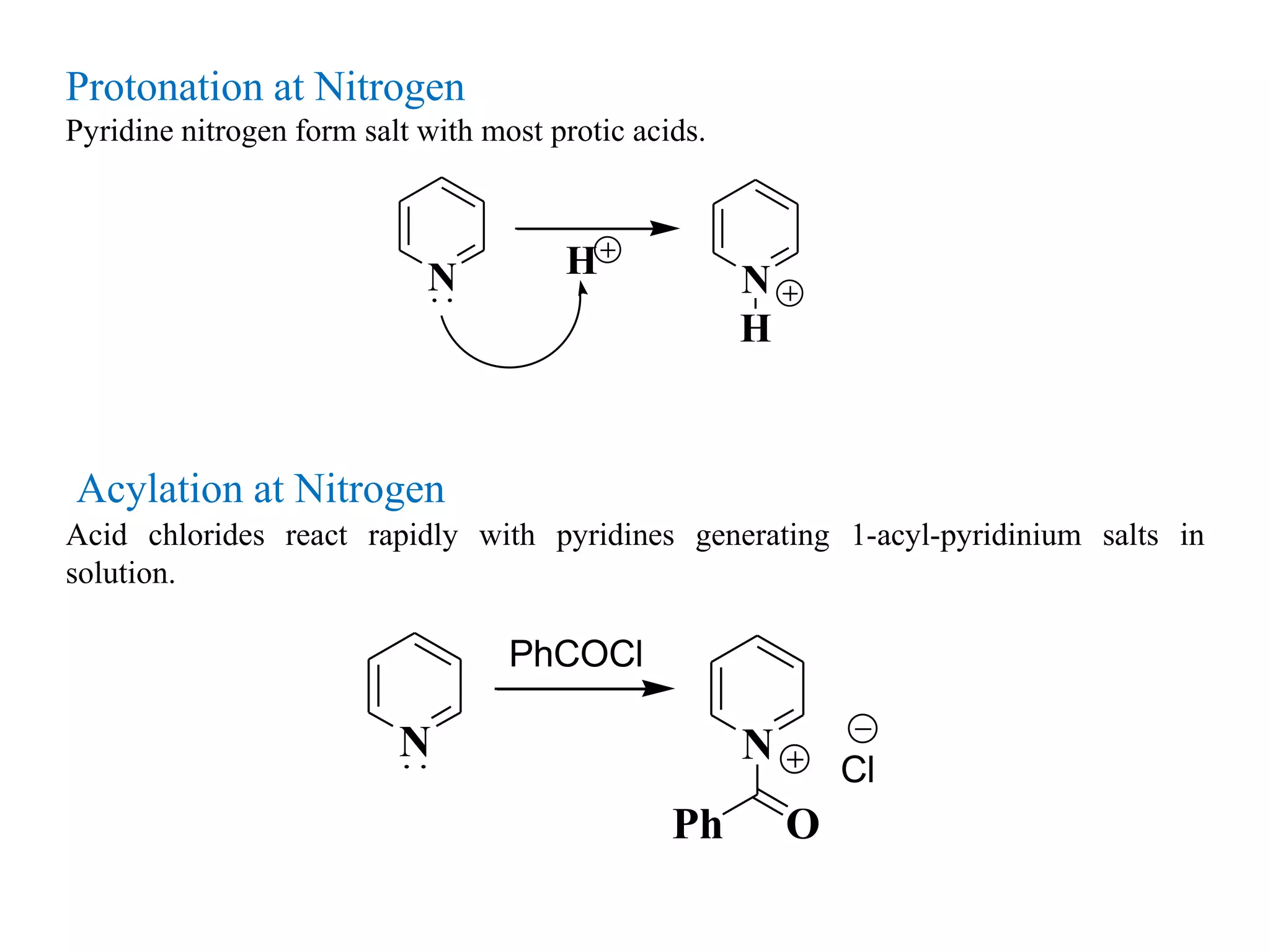
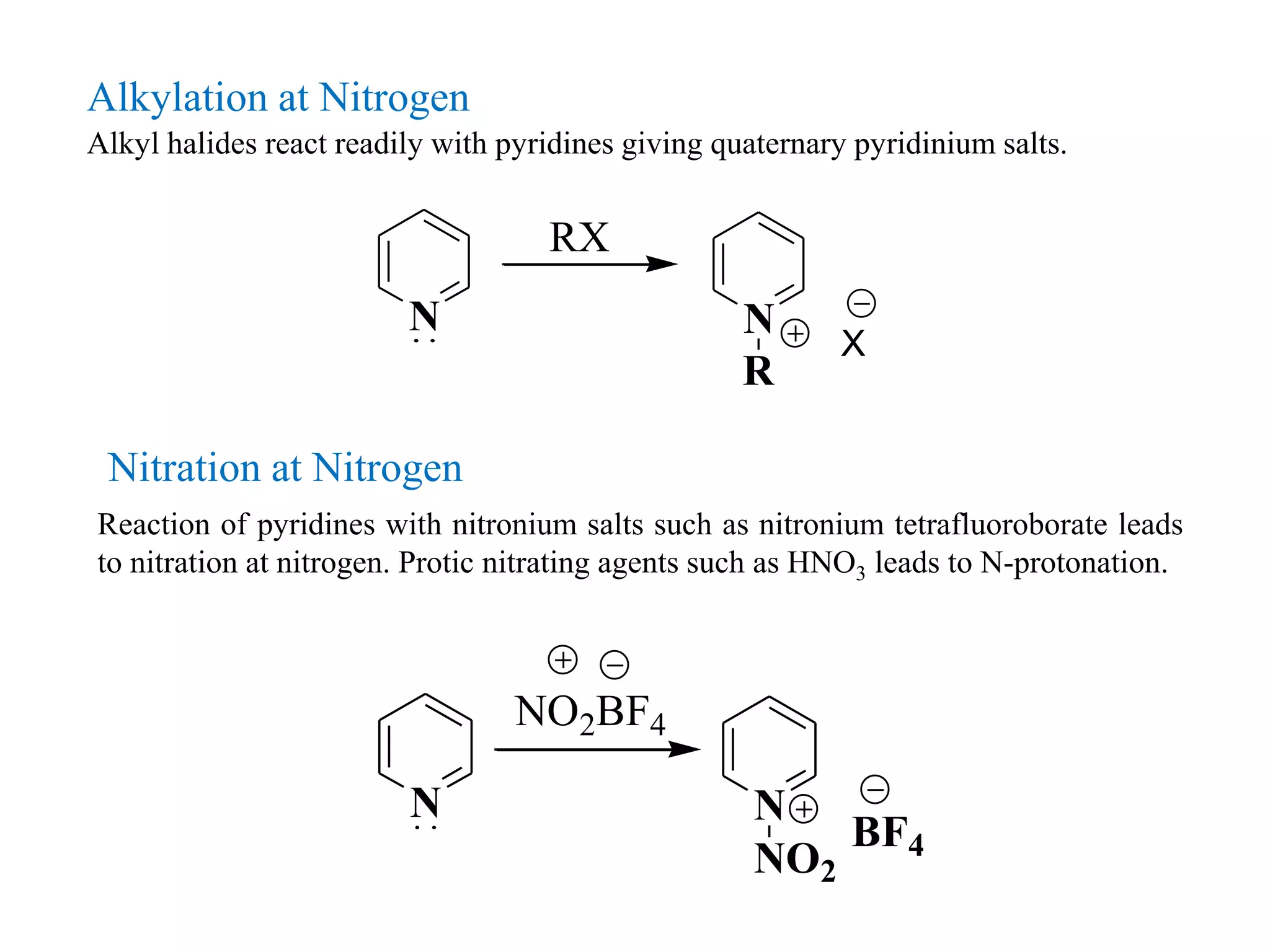
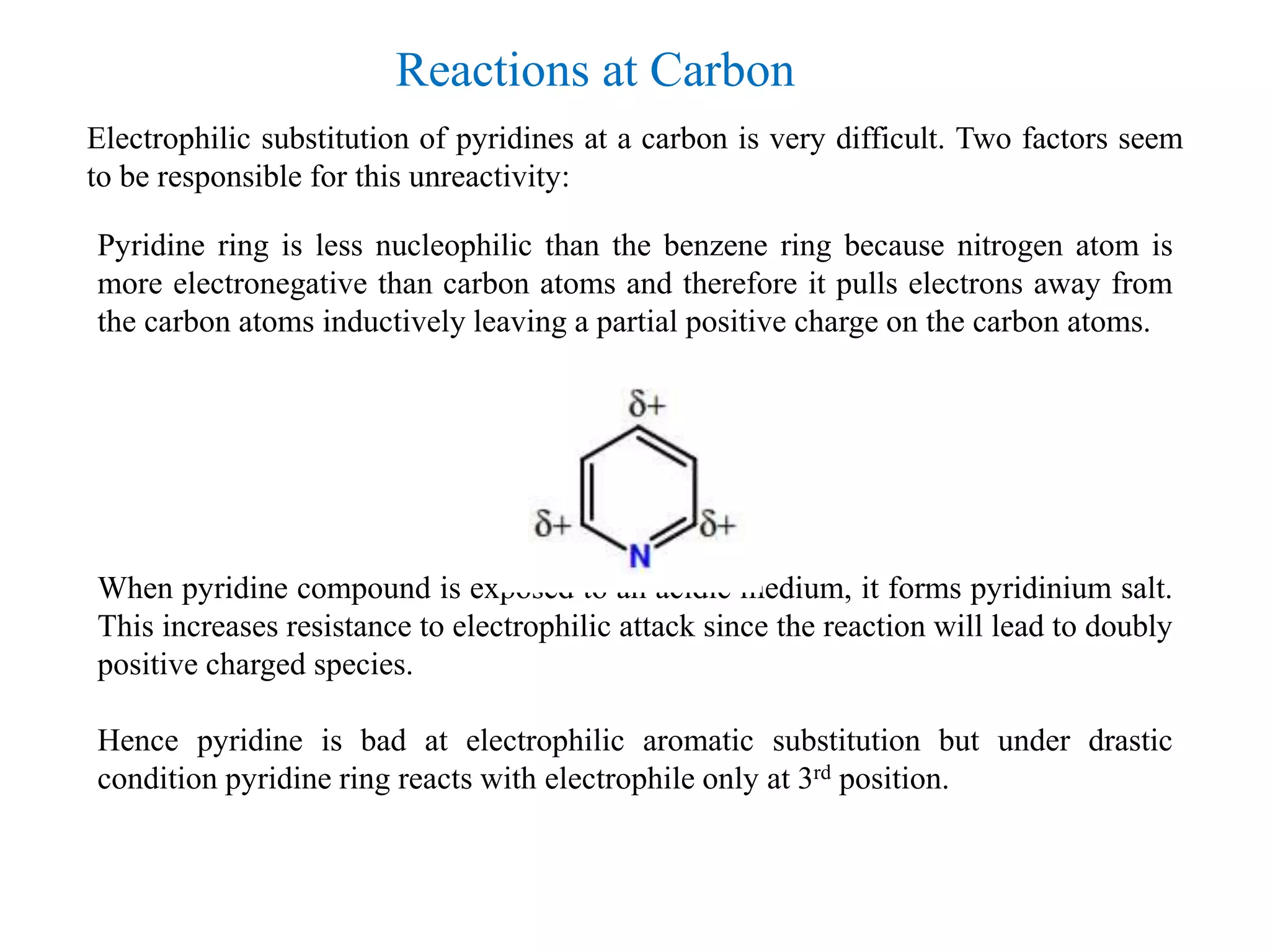
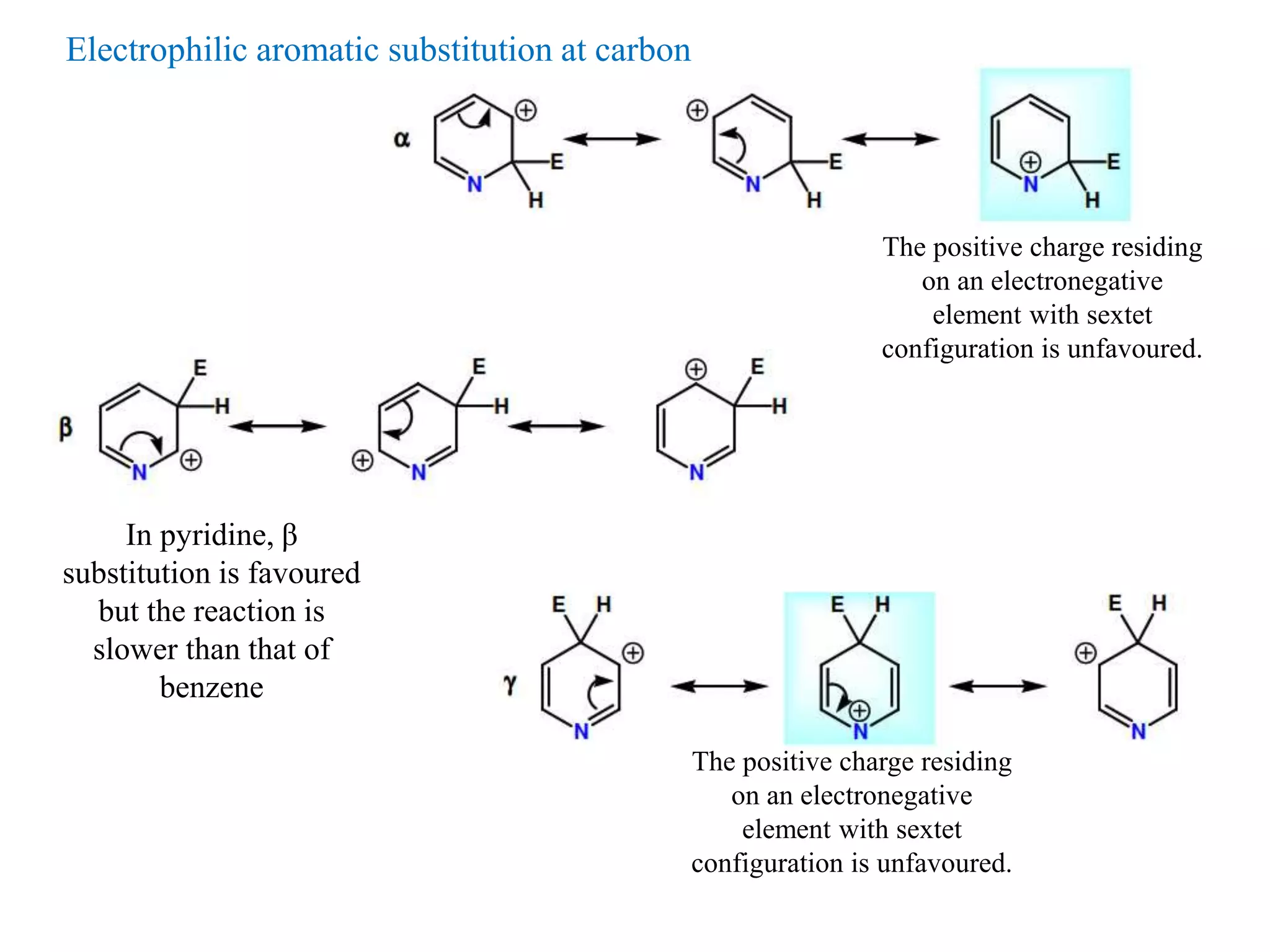
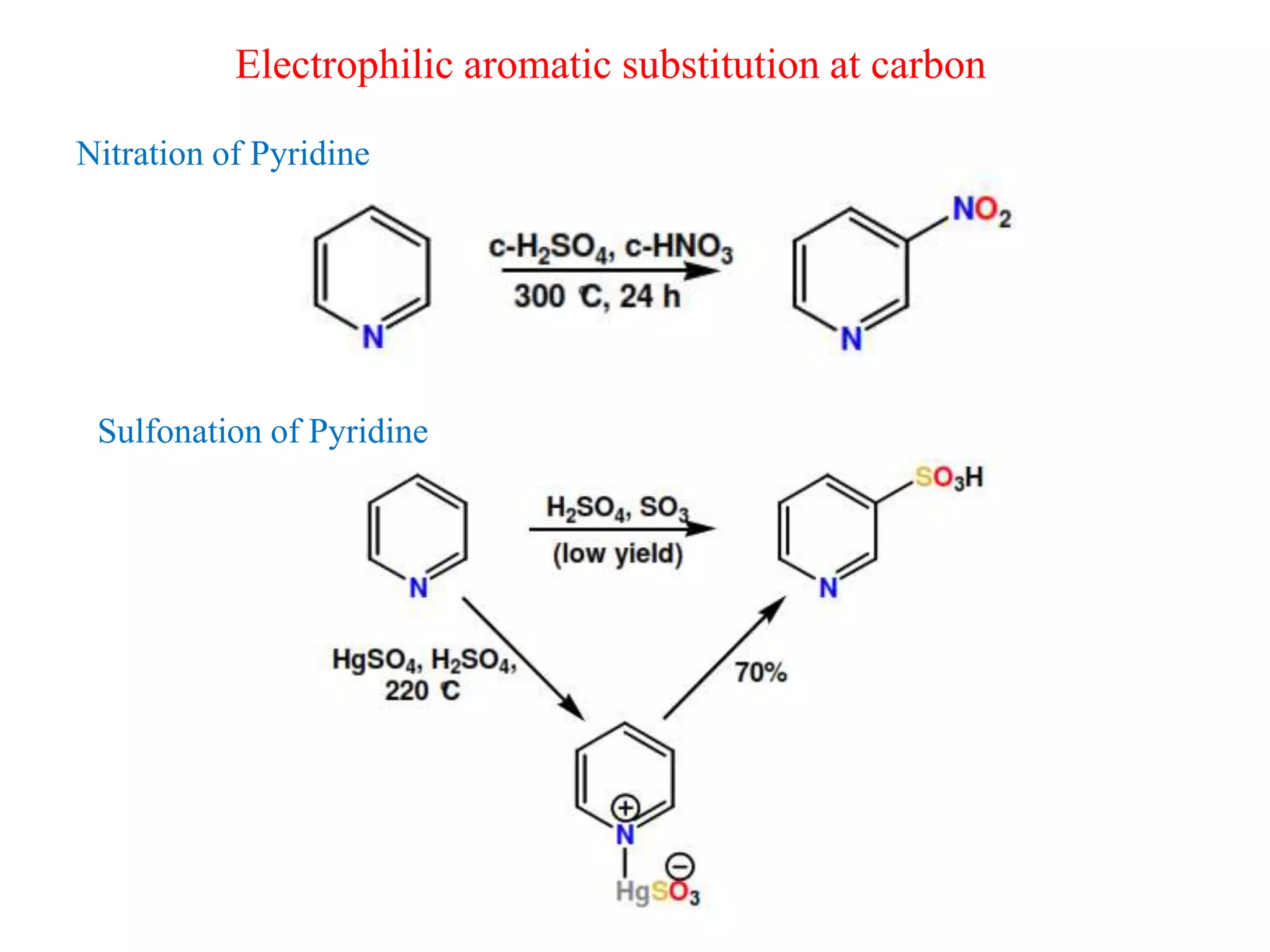
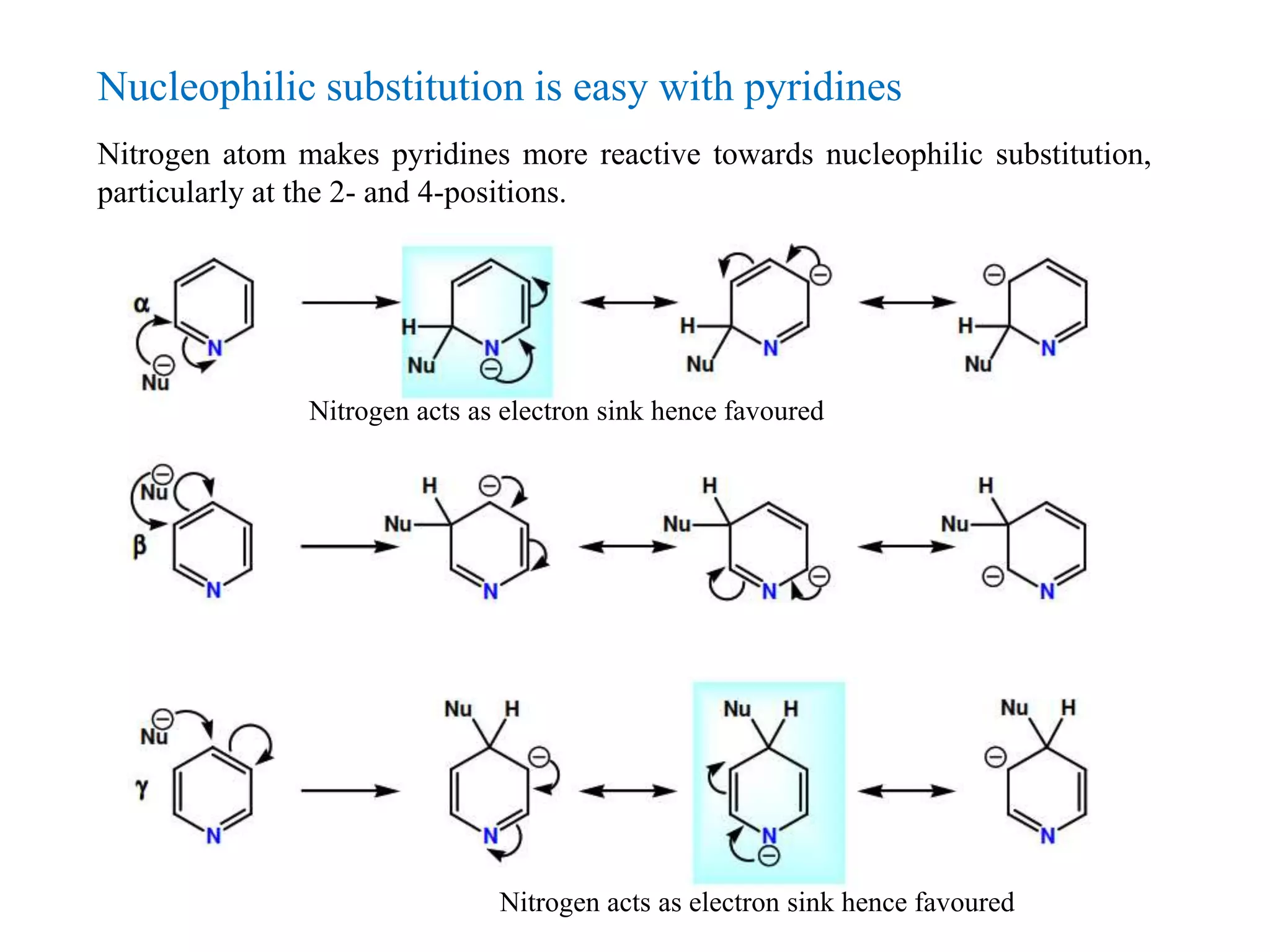
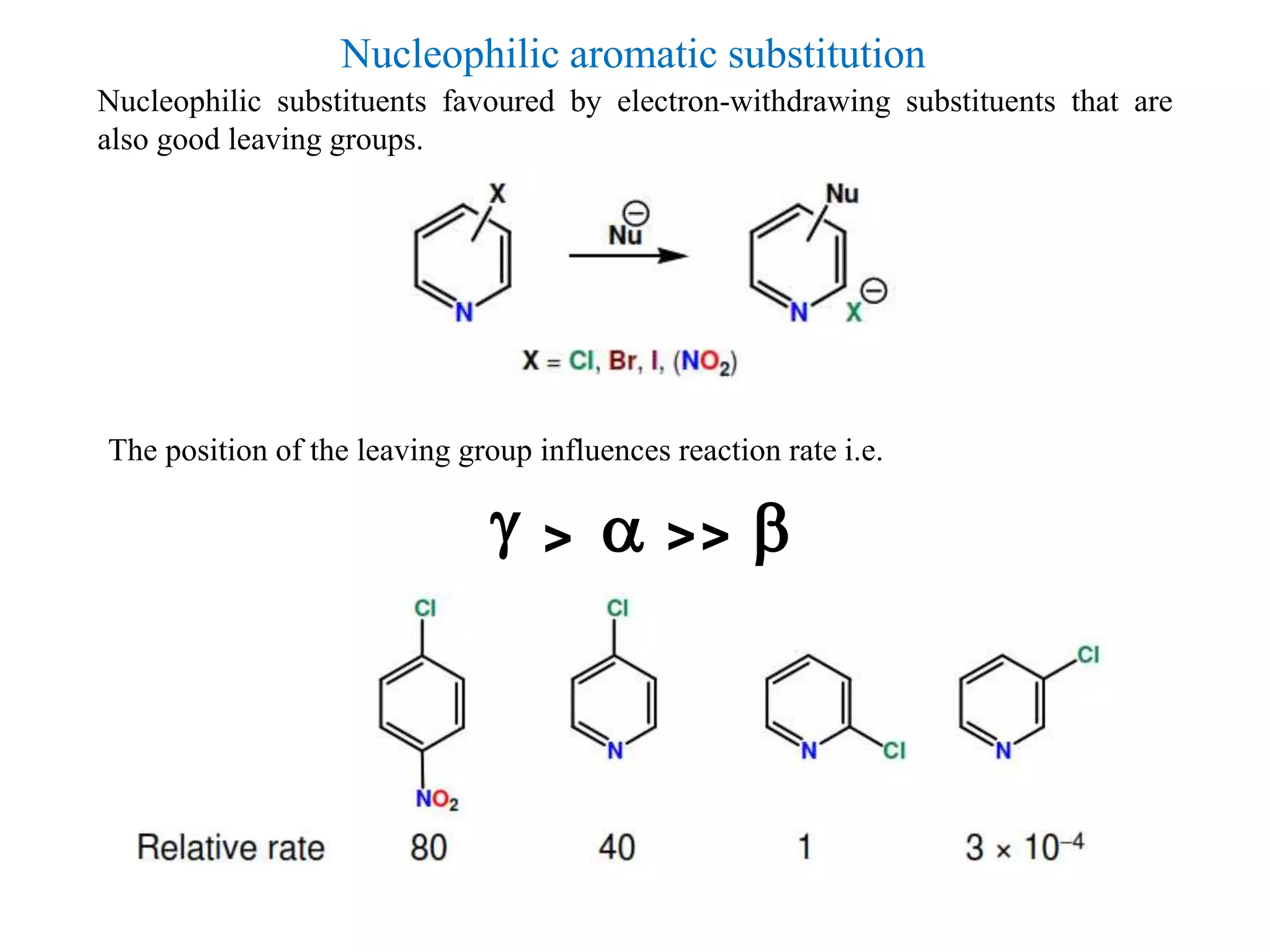
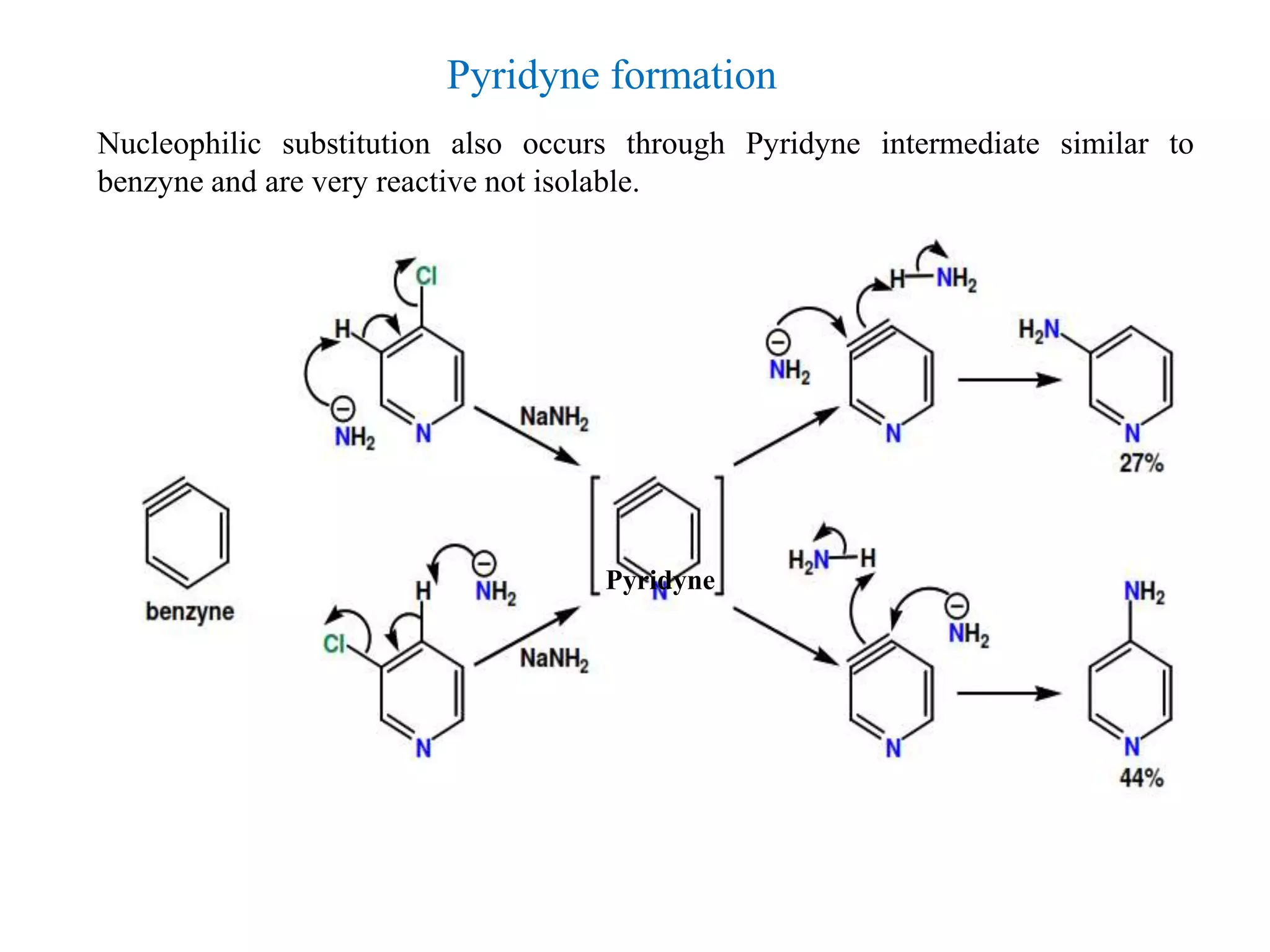
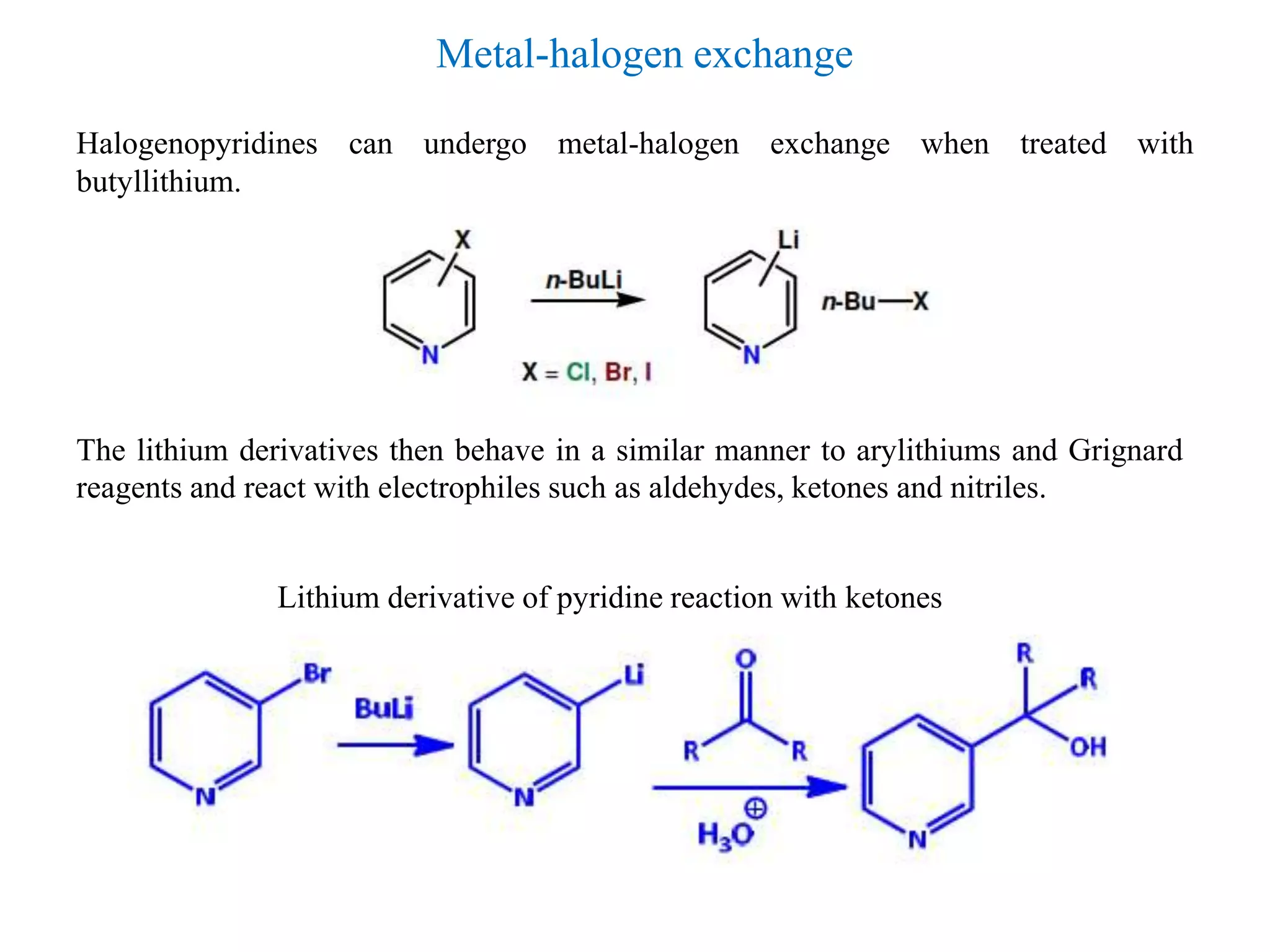
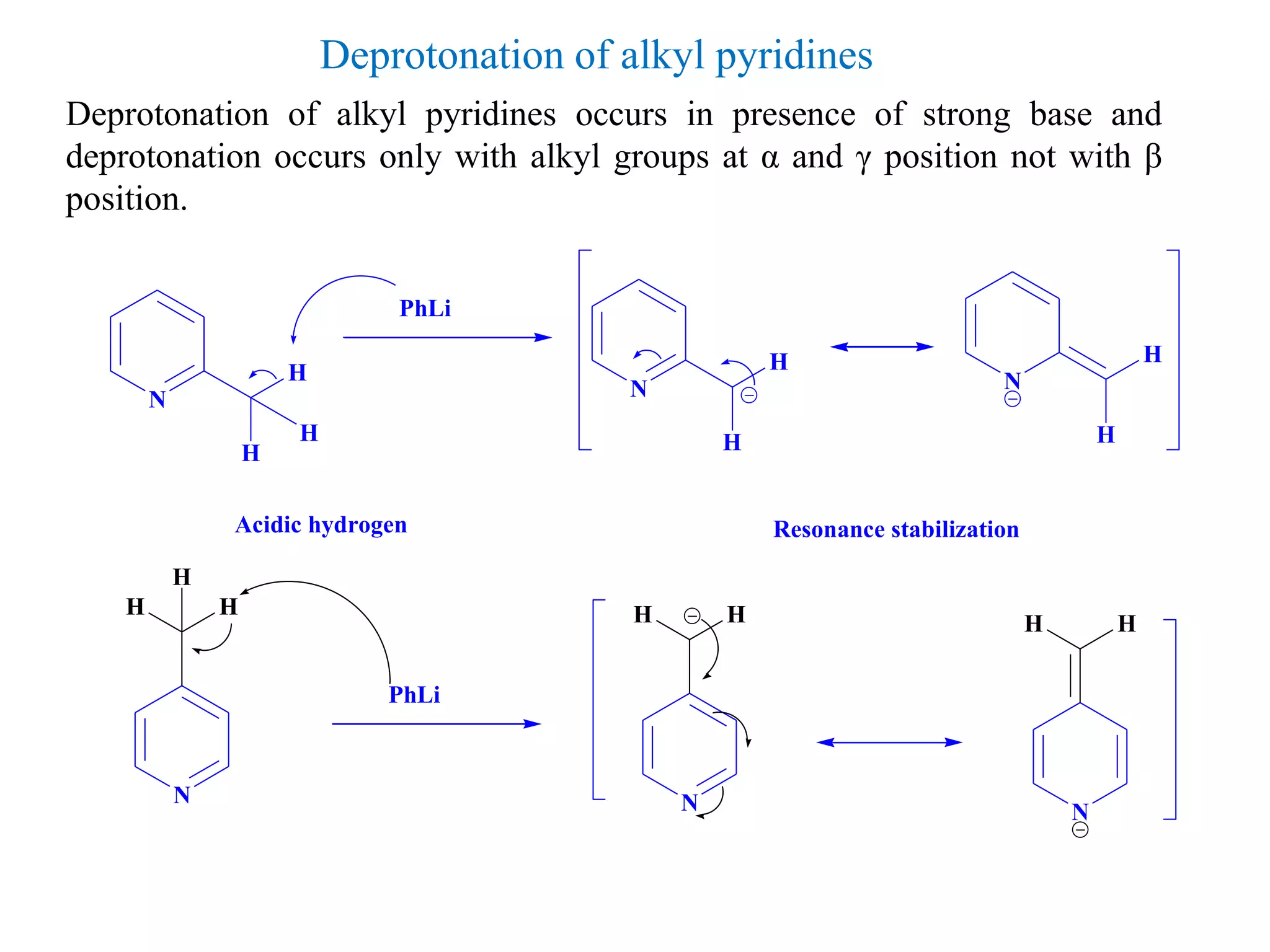
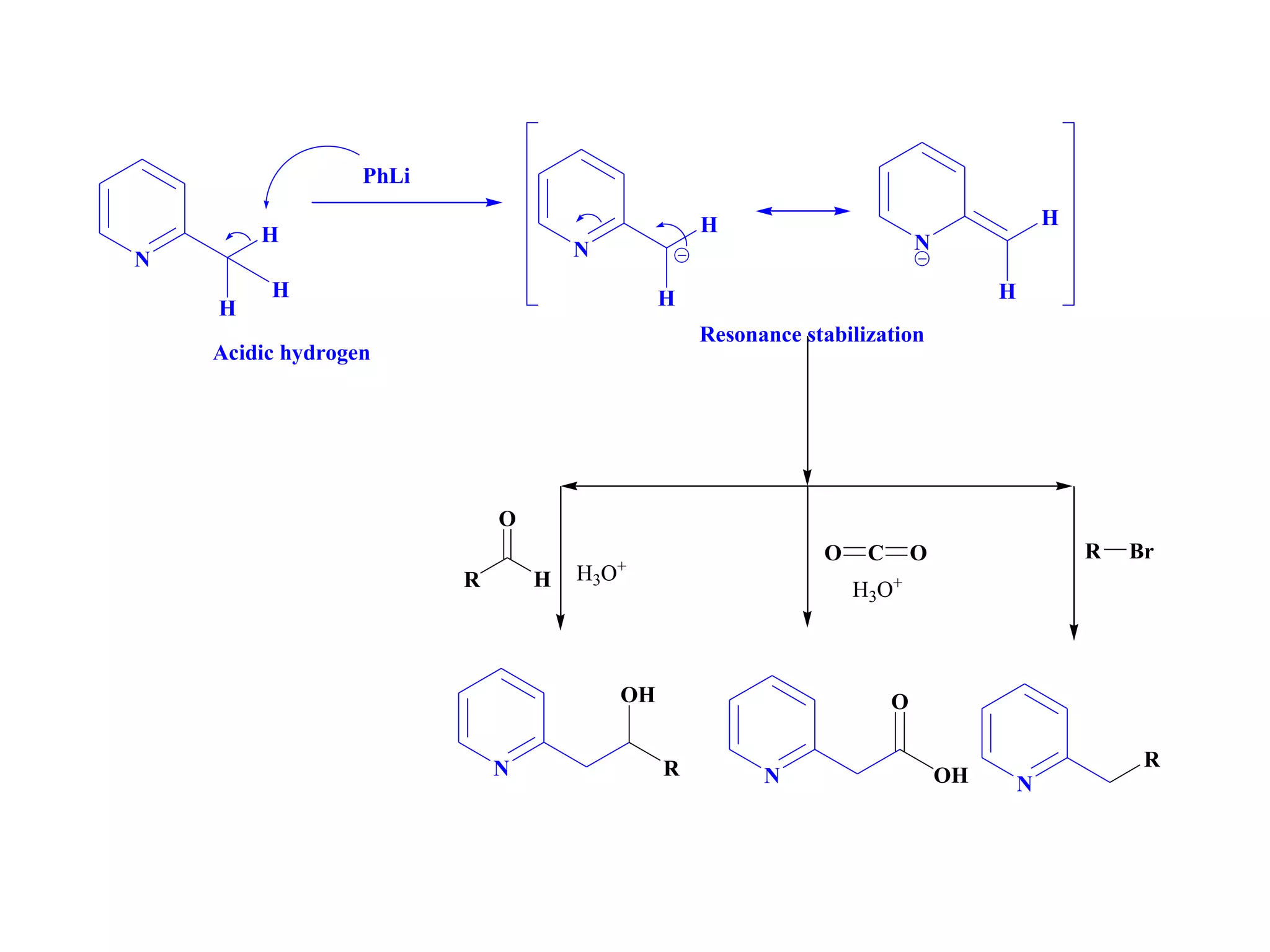
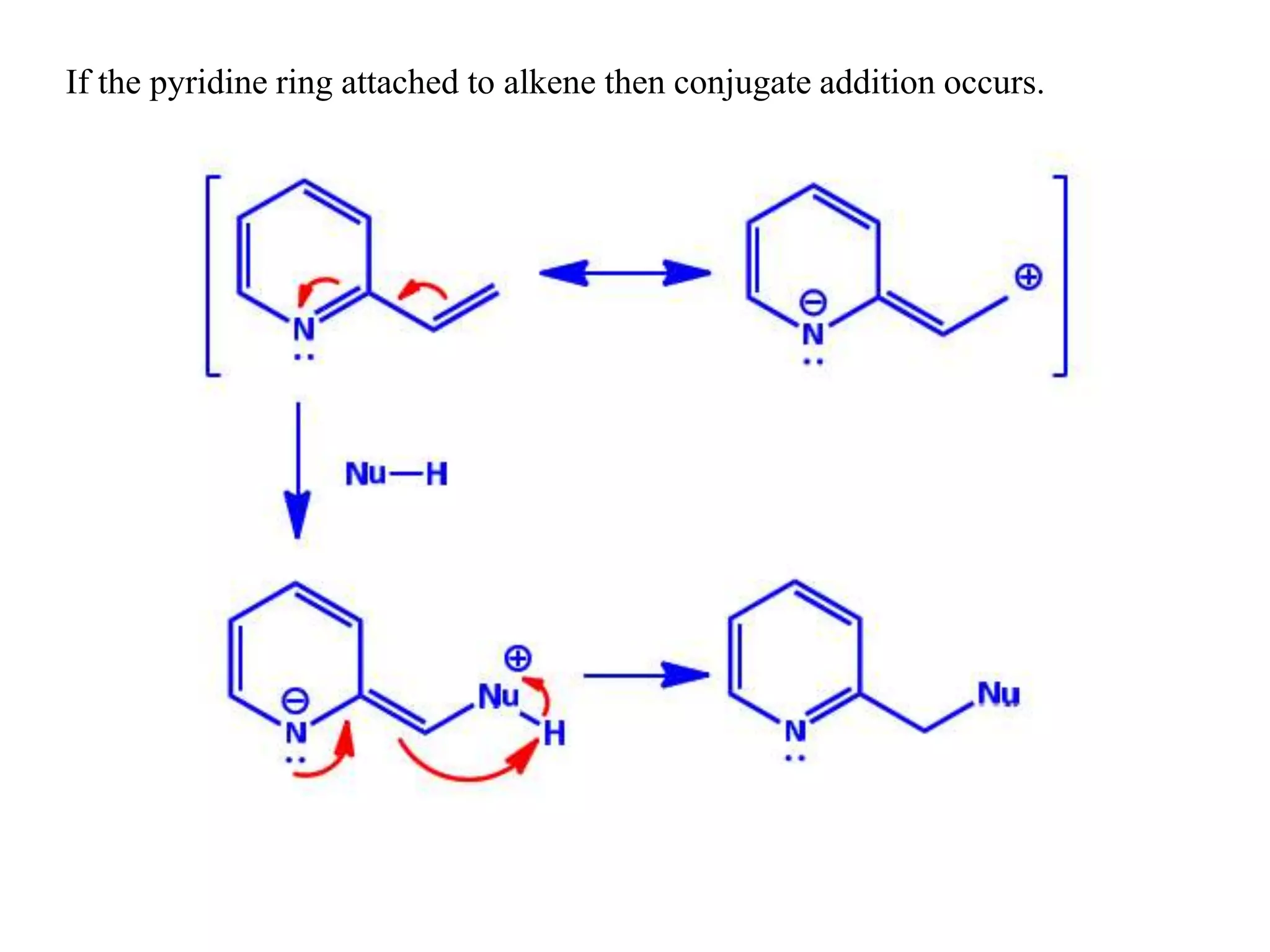
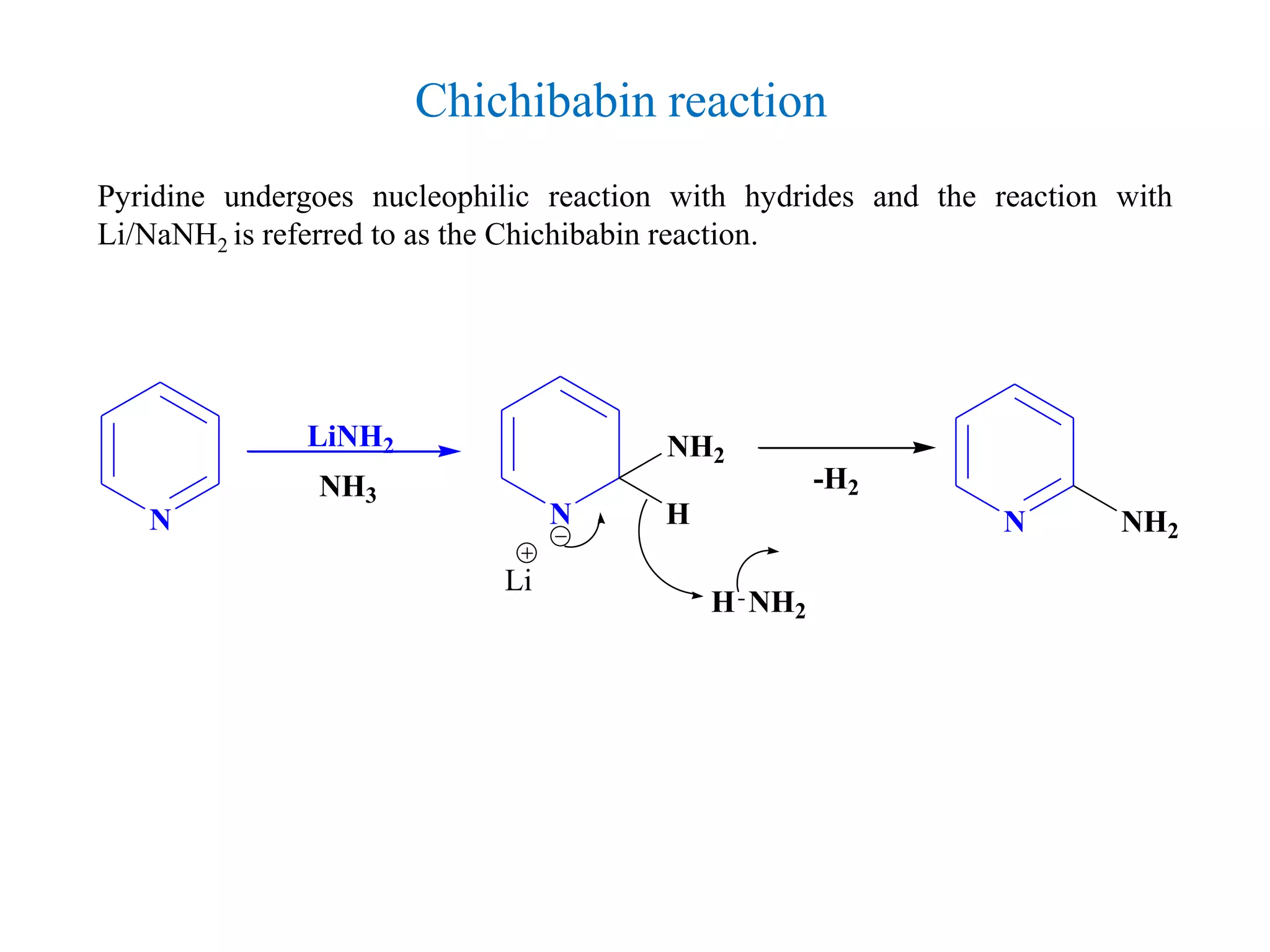
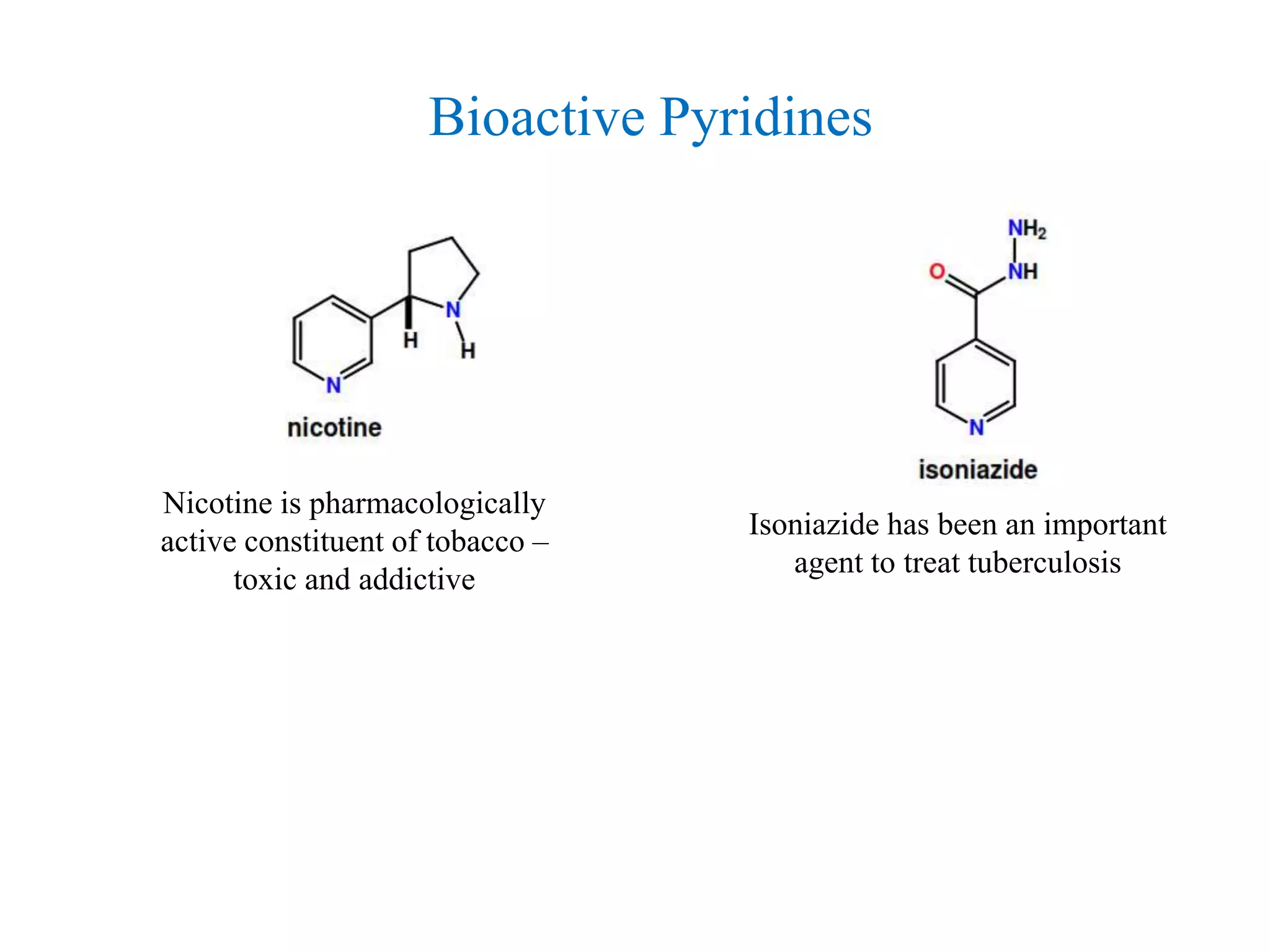
The document discusses the synthesis, reactivity, and significance of pyridine, a simple heterocycle derived from benzene involving nitrogen substitution. It outlines pyridine's basicity influenced by substituents, various synthesis methods including Hantzsch pyridine synthesis, and the reactivity of pyridine in electrophilic and nucleophilic substitution reactions. Additionally, it highlights specific derivatives of pyridine that are vital in medicinal chemistry, such as nicotine and isoniazid.