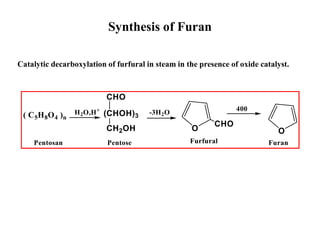
The document discusses aromatic five-membered heterocycles containing one heteroatom - pyrrole, furan, and thiophene. Pyrrole has a higher boiling point than furan and thiophene due to hydrogen bonding. These compounds undergo electrophilic substitution at the carbon atoms. Thiophene has the most resonance structures. The order of aromaticity is benzene > thiophene > pyrrole > furan. Pyrrole is acidic due to stabilization of its conjugate base by resonance. Furan can undergo Diels-Alder reactions while thiophene and pyrrole cannot due to aromaticity.



































![O
O
O
O
O
OHC
COCH3Ac2
O
1- DMF/POCl3
2- H
2 O
+ Na
2 CO
3
dil NHO3
CH3COOH,[0]
NO2
SO3 ,100
pyridine
HO3S
2-nitrofuran2-furan sulfonic acid
2-acetyl furae
furfulaldehyde
furfural BF3
NitrationSulfonation
Acetylation
Vilsmier Rx
Hetero-Monocyclic Compounds
Reactions of Furan](https://image.slidesharecdn.com/fivememberedheterocycliccompounds-200831153827/85/Five-membered-heterocyclic-compounds-37-320.jpg)

![Reactions of Thiophene
S
S
S
S
COCH3
CH3COCl
dil NHO3
CH3COOH,[0]
NO2
SO
3 , 100pyridine
HO3S
2-thiophene-2- sulfonic acid
SOCL4
S
NO
2O2NHNO3
SS
S S S
Br
Br
Br
Cl Cl
Cl
Cl
Cl Cl
Cl
Br 2
AcOH
Cl2
50
major minor
Nitration
Sulfonation
Acetylation
Halogenation
Hetero-Monocyclic Compounds](https://image.slidesharecdn.com/fivememberedheterocycliccompounds-200831153827/85/Five-membered-heterocyclic-compounds-39-320.jpg)



![Hetero-Monocyclic Compounds
The order of aromaticity
Benzene > Thiophene > Pyrrole > Furan
In case of Thiophene [S]
donate & accept electrons……
so delocalization as complete as benzene
S
In case of Furan [O]
electronegativity more.
Diene-like character CH2=CH-CH=CH2
O](https://image.slidesharecdn.com/fivememberedheterocycliccompounds-200831153827/85/Five-membered-heterocyclic-compounds-43-320.jpg)


































