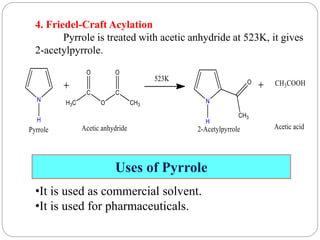
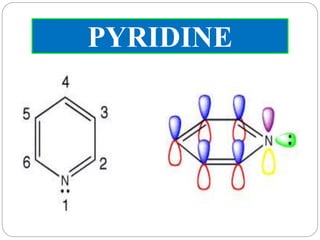
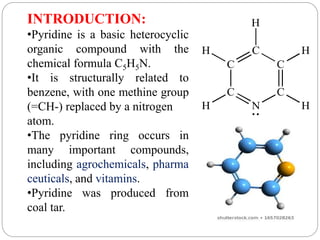
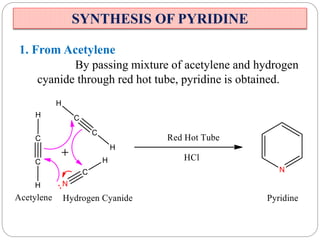
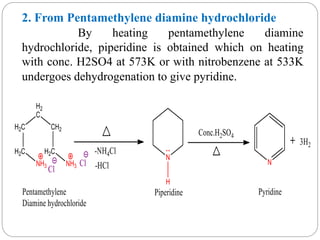
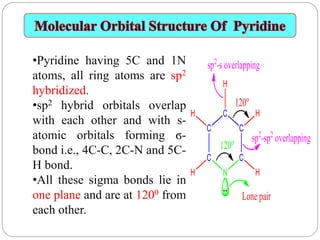
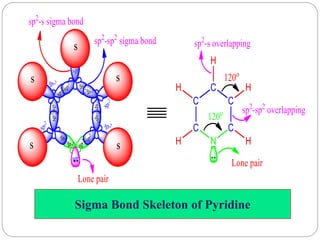
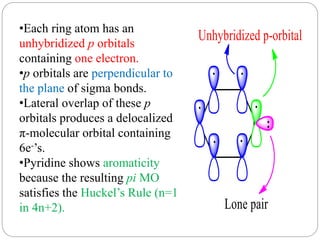
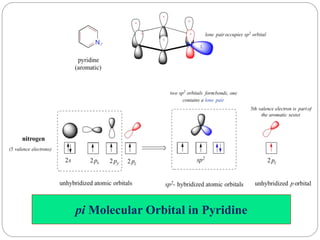
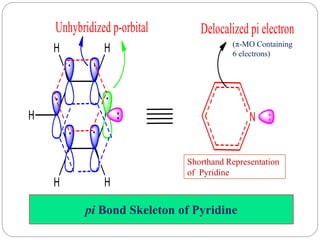
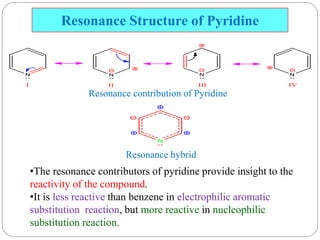
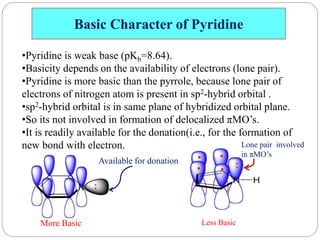
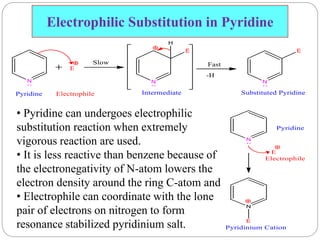
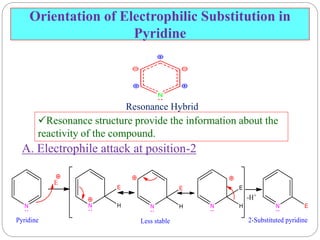
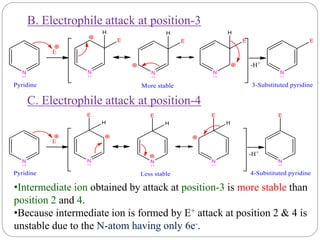
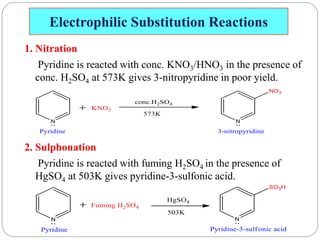
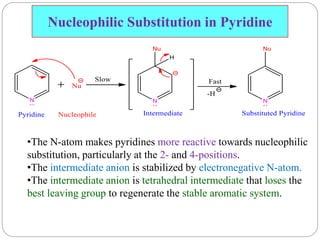
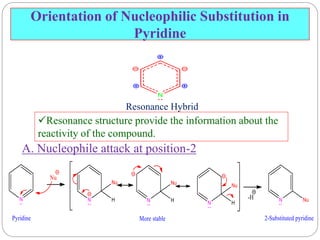
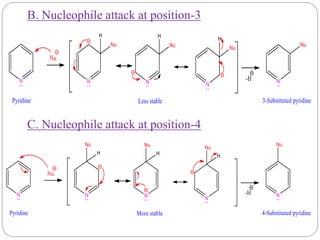
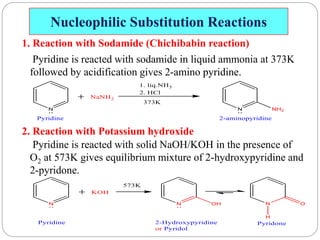
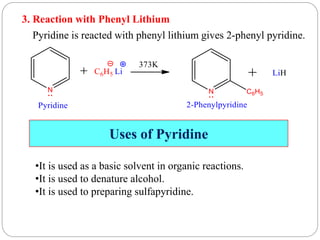
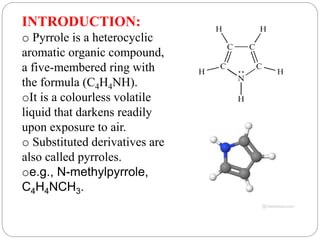
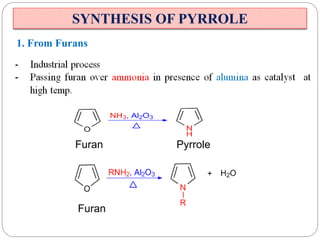
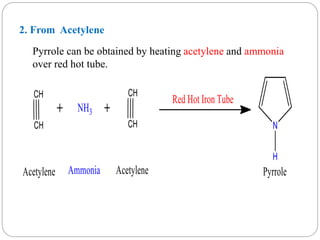
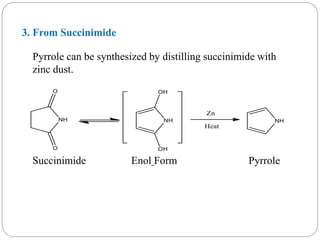
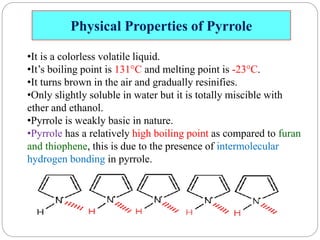
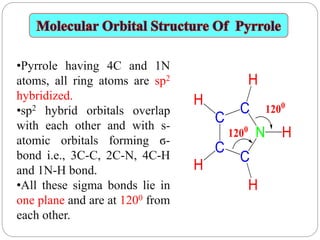
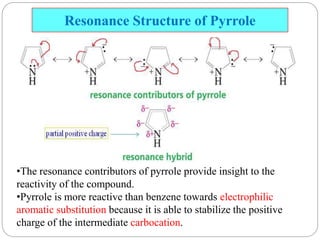
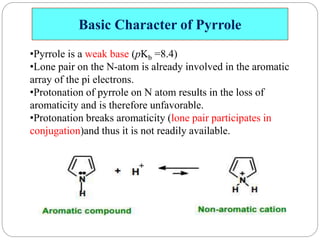
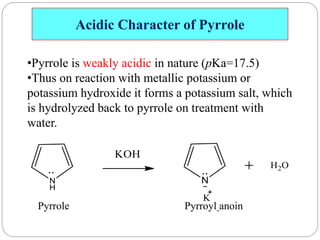
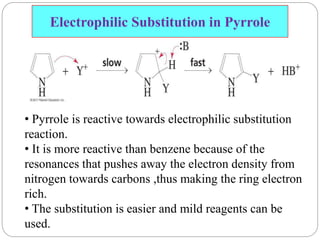
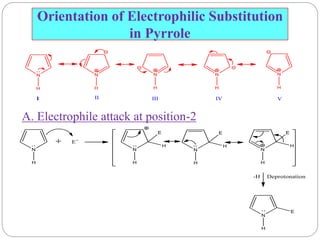
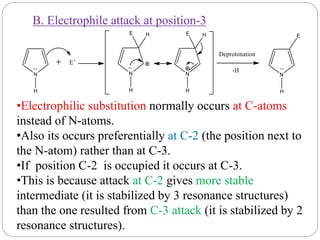
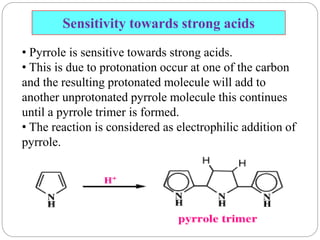
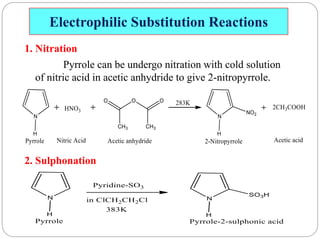
The document discusses the organic chemistry of heterocyclic compounds, particularly focusing on pyrrole and pyridine. It outlines their nomenclature, synthesis, physical properties, and reactivity, including their electrophilic and nucleophilic substitution reactions. It emphasizes the similarities and differences between the two compounds, as well as their applications in pharmaceuticals and as solvents.
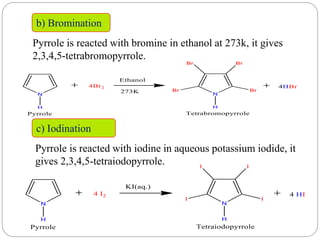
![3. Halogenation
a) Chlorination
i] Pyrrole is reacted with sulphuryl chloride in ether at
273K gives 2,3,4,5-tetrachloropyrrole.
ii] Pyrrole is reacted with chlorine gives 1,2,3,4,5-
pentachloropyrrole.](https://image.slidesharecdn.com/unit-iii-heterocycliccompounds-220902155505-c050eceb/85/Unit-III-Heterocyclic-Compounds-pptx-27-320.jpg)