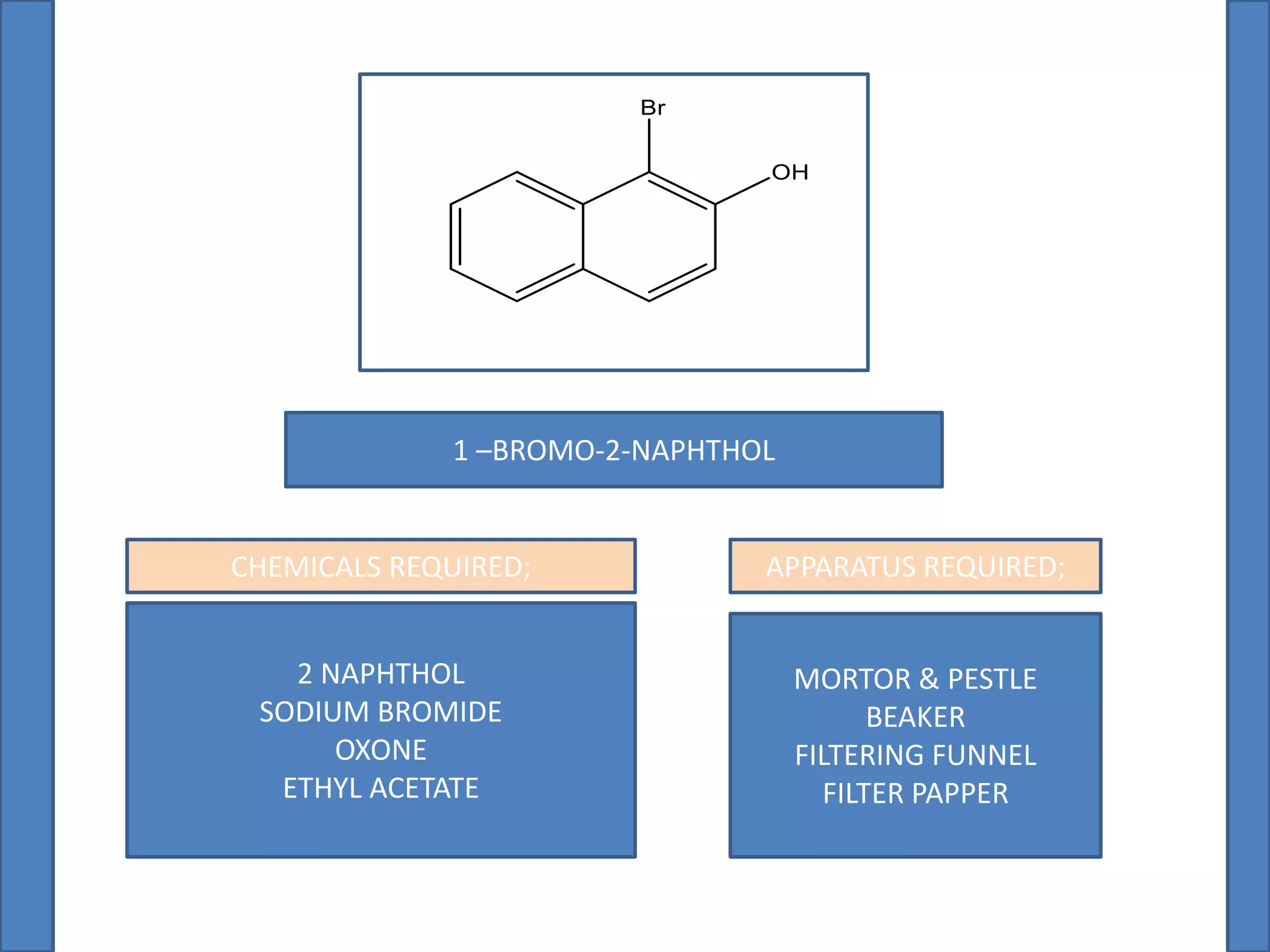
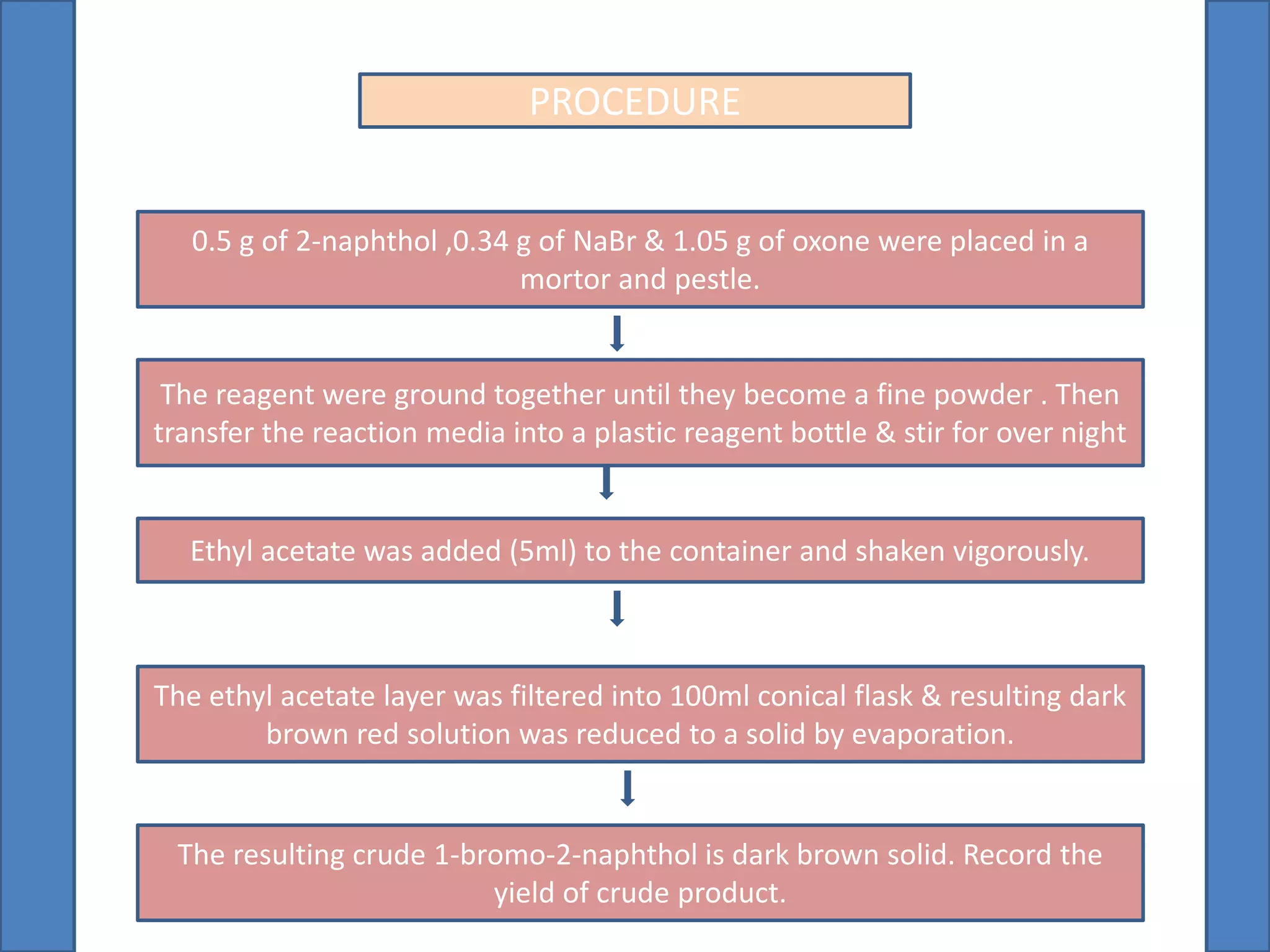
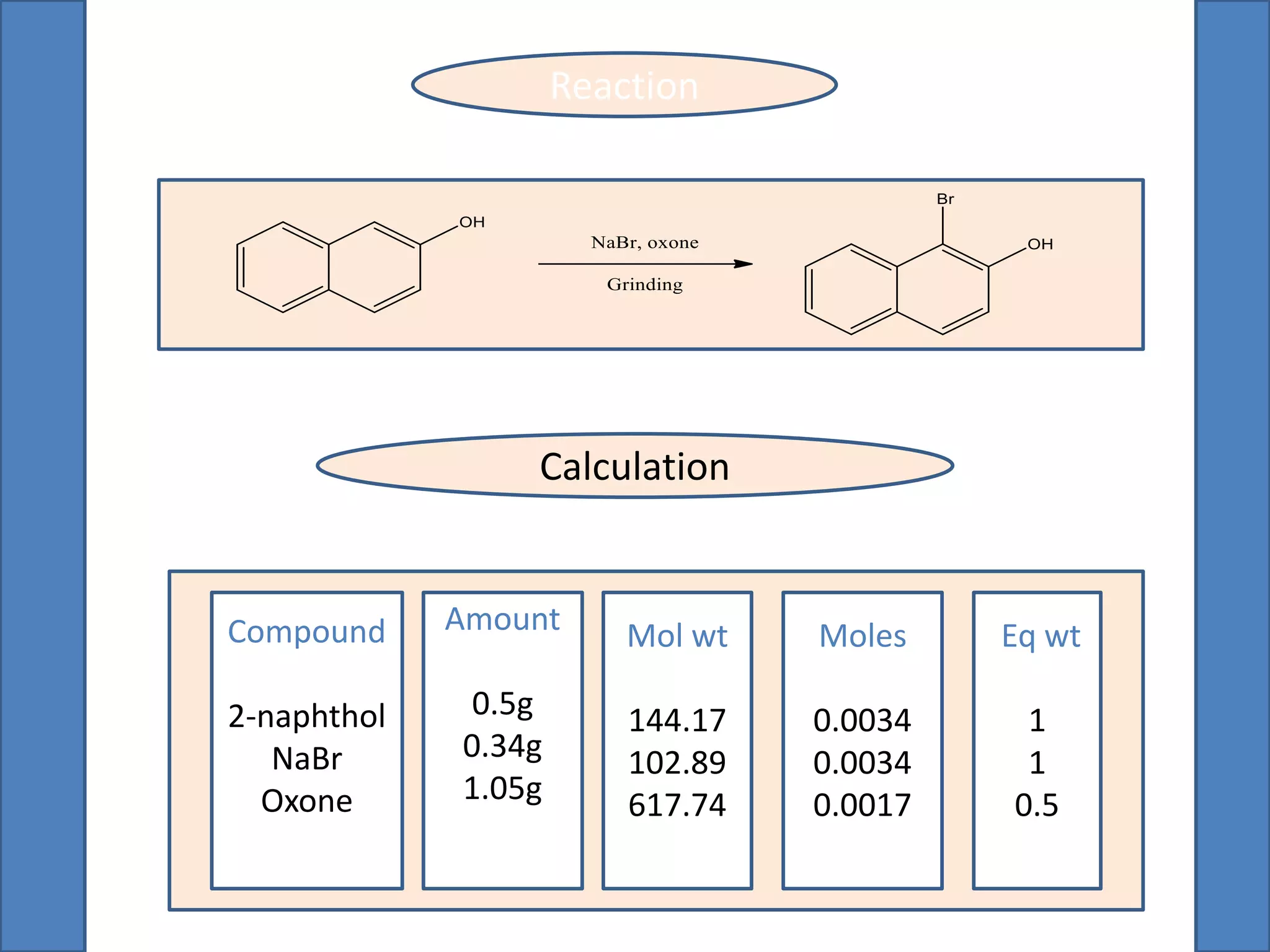
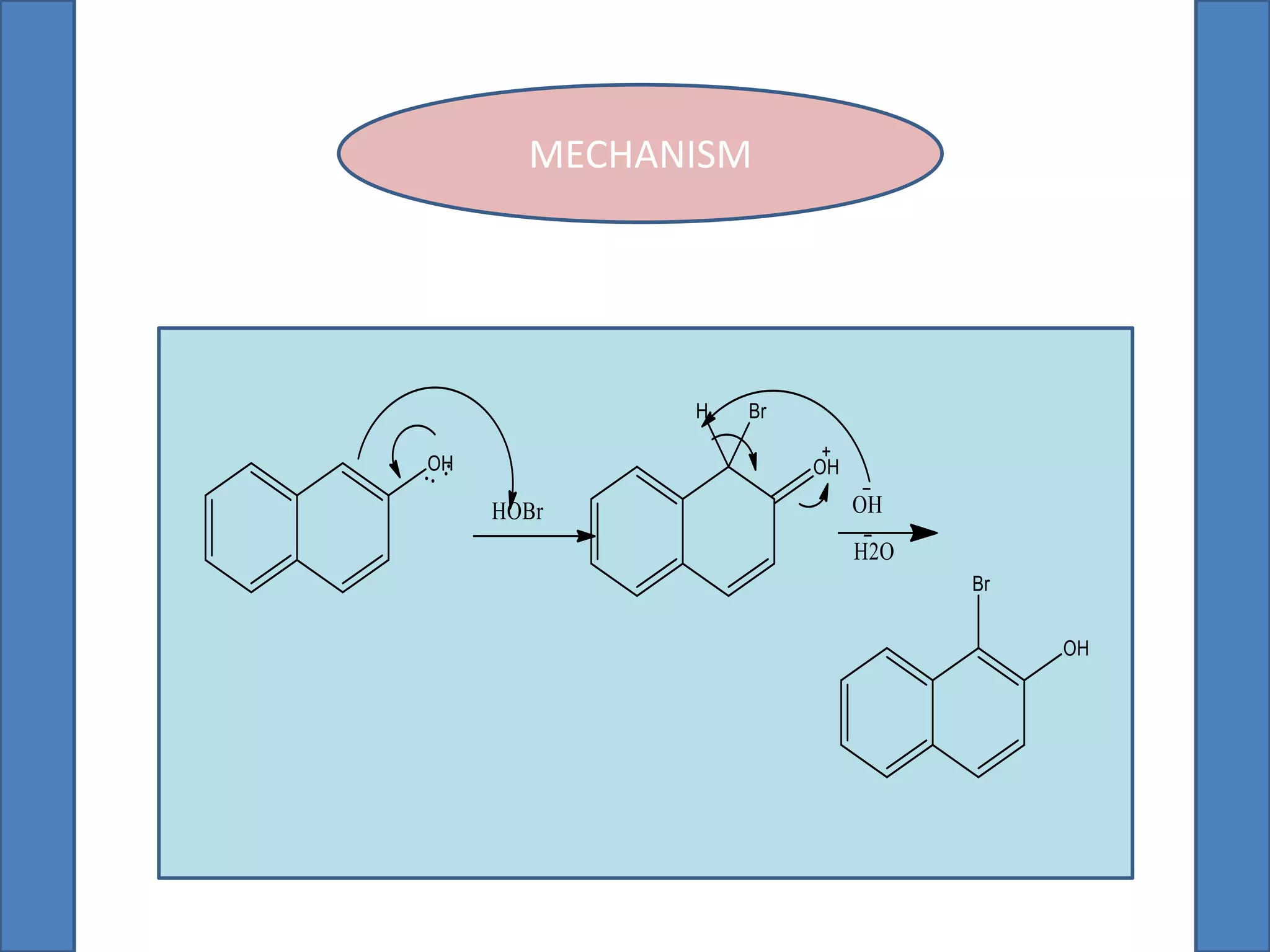
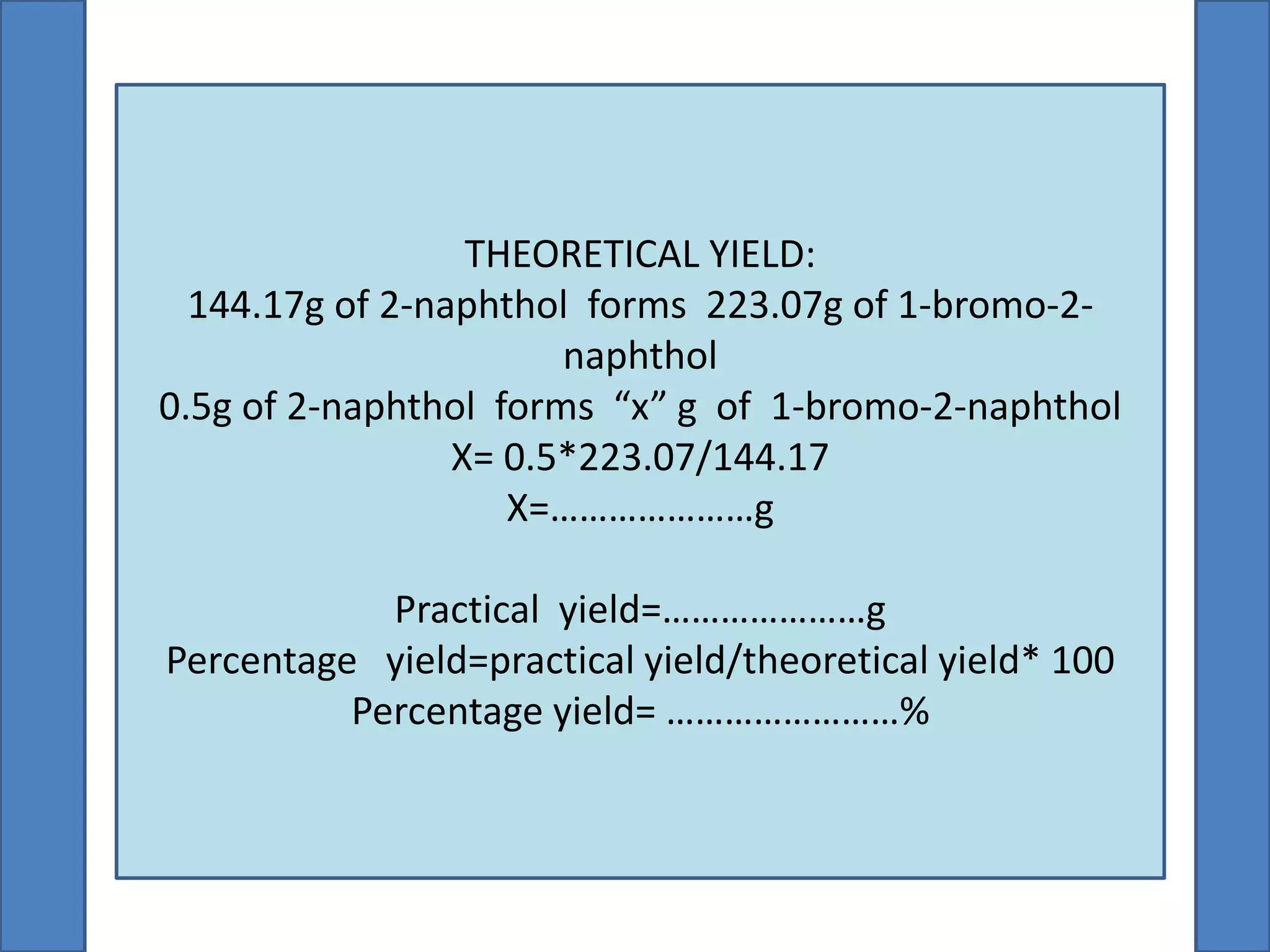
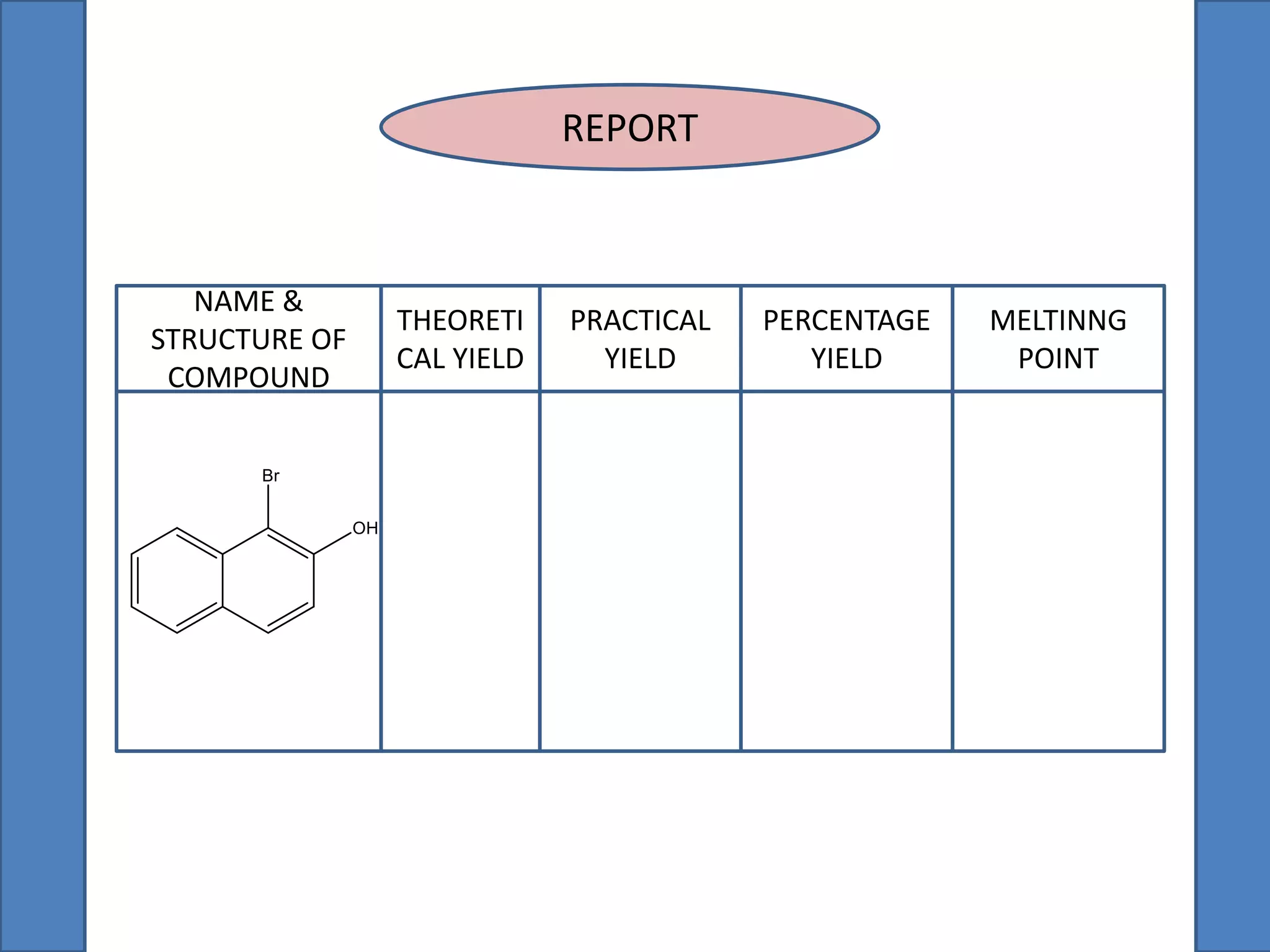
This document summarizes the synthesis of 1-bromo-2-naphthol from 2-naphthol. It involves selectively brominating 2-naphthol using sodium bromide and oxone. 2-Naphthol, sodium bromide, and oxone are ground together and reacted overnight. Ethyl acetate is then used to extract the crude 1-bromo-2-naphthol product, which is a dark brown solid. The theoretical and practical yields are calculated and the percentage yield is reported.