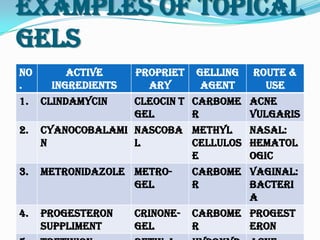
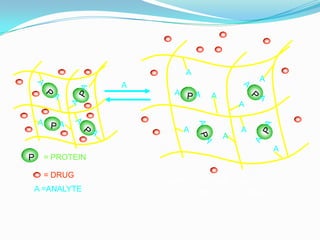
This document provides an overview of pharmaceutical gels. It defines gels as semisolid colloidal systems where a liquid vehicle interacts with colloidal particles. The vehicle can be aqueous, hydroalcoholic, alcoholic, or non-aqueous. Gels are classified based on their continuous phase (organogels, hydrogels, xerogels) or the nature of bonds in their 3D network (dispersed solids, hydrophilic polymers). Common gelling agents include natural polymers, semisynthetic polymers, and synthetic polymers. The document discusses gel properties, preparation methods, manufacturing parameters, examples of topical gels, and applications of gels in drug delivery.