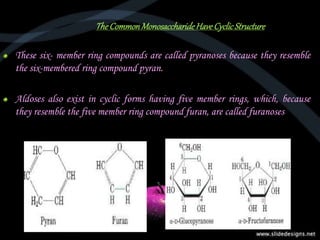
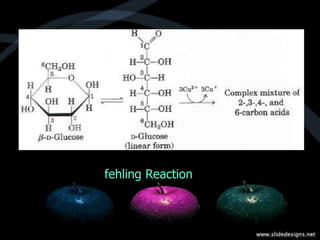
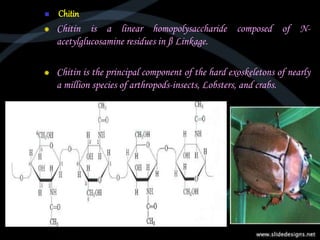
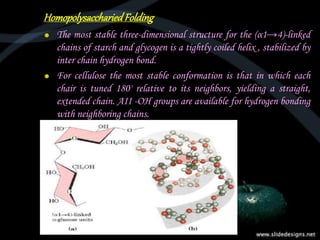
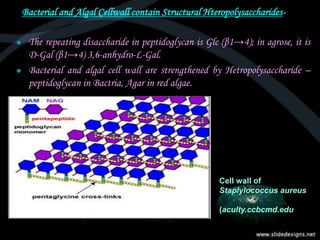
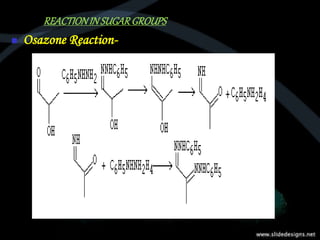
This document provides an overview of carbohydrates, including classifications such as monosaccharides, disaccharides, and polysaccharides, and their specific structures and functions. Monosaccharides are simple sugars with cyclic forms, while polysaccharides serve various roles, including storage (starch and glycogen) and structural functions (cellulose and chitin). The document also discusses sugar reactions and references various biochemical sources.