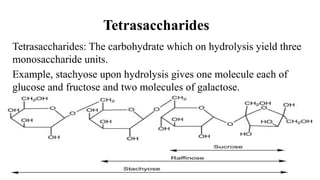
The document provides a detailed overview of carbohydrates, including their classification into monosaccharides, oligosaccharides, and polysaccharides, as well as their chemical structures and functions in energy provision, storage, and structural roles in cells. Key examples include glucose, sucrose, starch, glycogen, cellulose, and heparin, with explanations about their properties, significance, and some health-related effects. The document highlights the importance of carbohydrates in both biological systems and various commercial applications.



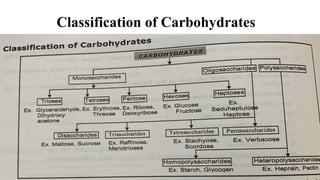
![Oligosaccharides
Greek word - oligo = few; sacchar sugar] oligosaccharides are composed of 2-
10 simple sugars (monosaccharides).
Depending upon the presence of monosaccharide units, these are further
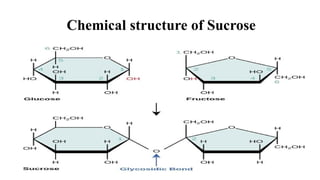
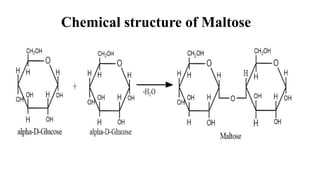
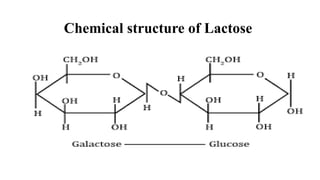
divided as:Dísaccharides:
These sugars consist of two monosaccharide units joined together with
glycosidic bond.
Example: sucrose, maltose, lactose,](https://image.slidesharecdn.com/carbohydratesintroductionfunctionsandclassification-240925184125-cf32f9bb/85/Carbohydrates-Introduction-Functions-and-Classification-pptx-8-320.jpg)



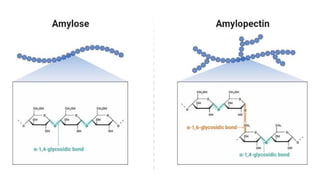
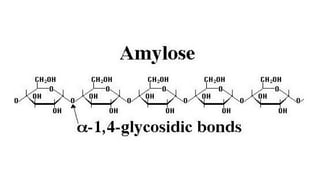
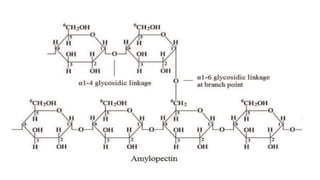
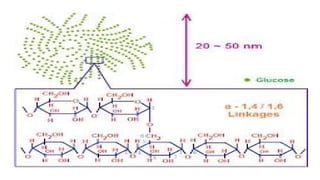
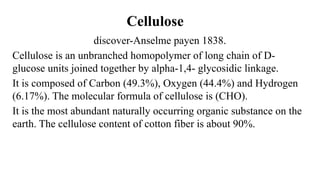
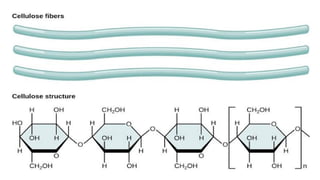
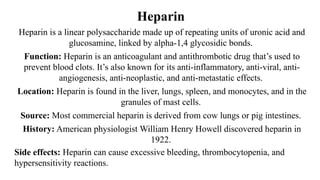
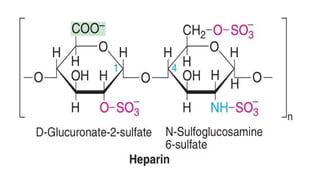
![Polysaccharides
[Greek poly many; sacchar-sugar] Polysaccharides consist of long chains of
same or different monosaccharide units linked together by glycosidic bond.
They may be tasteless, amorphous and insoluble in water.
Their structures may be linear to highly branch.
They are further classified as:
(a) Homopolysaccharides: Polysaccharides composed of the same
monosaccharide units are called homopolysaccharide.
Examples: starch, glycogen, cellulose.
(b) Heteropolysaccharides: Polysaccharides composed of different
monosaccharide units are called heteropolysaccharide. Example: heparin.](https://image.slidesharecdn.com/carbohydratesintroductionfunctionsandclassification-240925184125-cf32f9bb/85/Carbohydrates-Introduction-Functions-and-Classification-pptx-17-320.jpg)