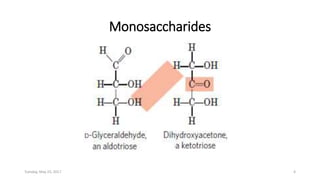
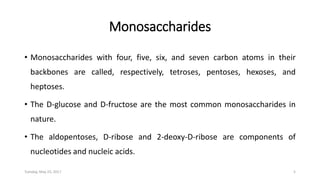
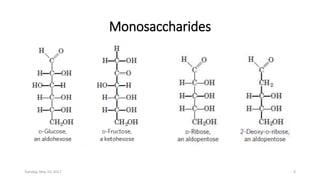
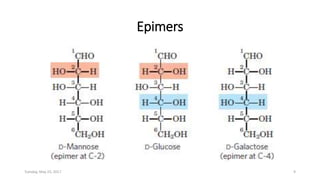
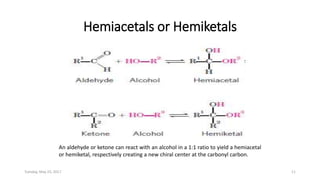
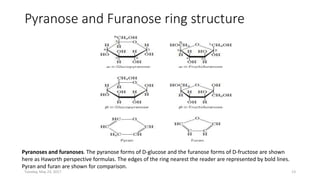
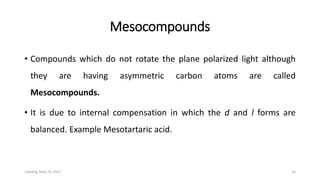
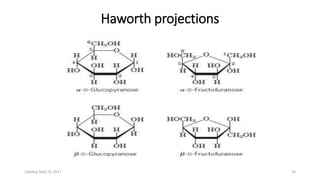
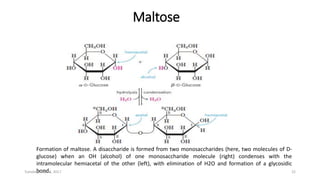
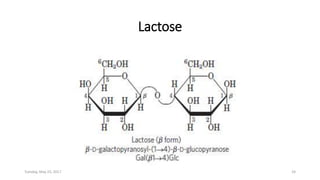
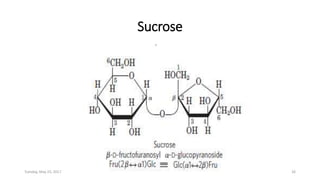
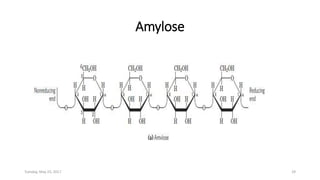
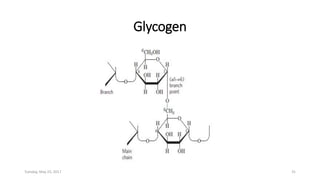
Carbohydrates are an important class of biological molecules. They include monosaccharides, disaccharides, and polysaccharides. Monosaccharides are the simplest form and include glucose, fructose, and ribose. Many monosaccharides exist as ring structures in aqueous solution through hemiacetal or hemiketal linkages. Disaccharides are formed from two monosaccharide units and include maltose, lactose, and sucrose. Polysaccharides serve important structural and storage functions and include starch, cellulose, and glycogen. Carbohydrates play critical roles in energy storage, structure, and numerous biological processes.