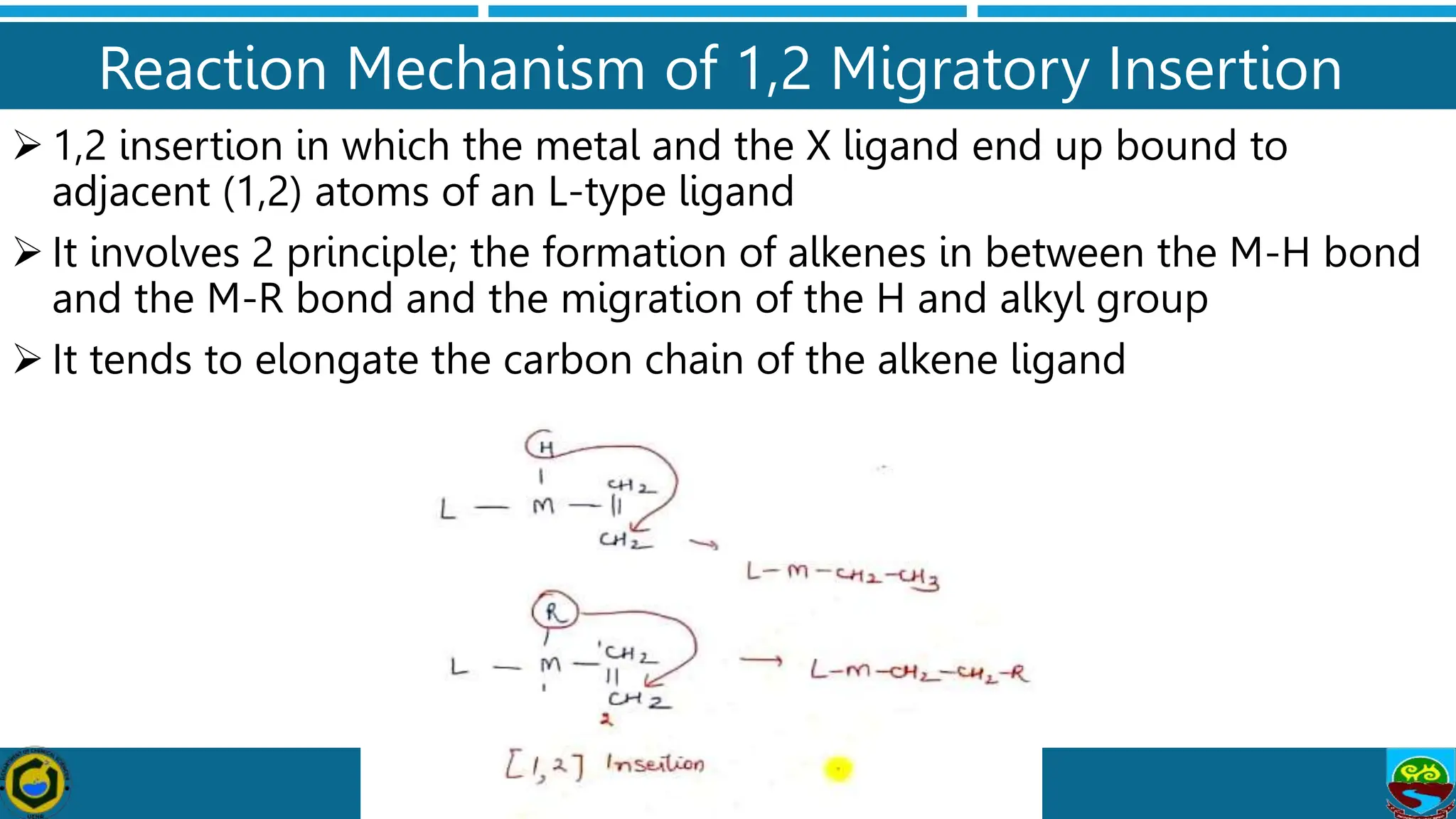
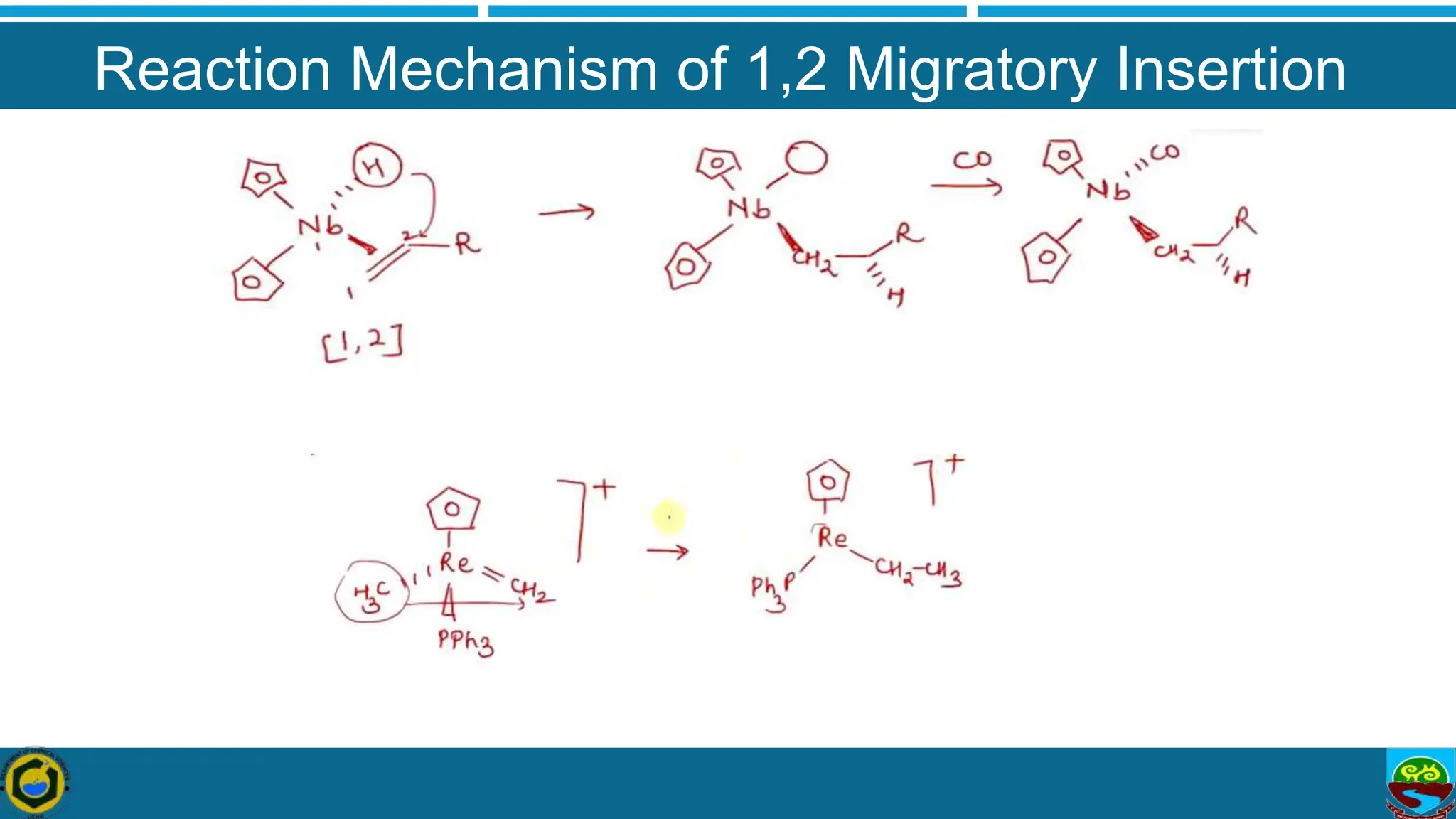
The document discusses migratory insertion reactions involving organometallic complexes, detailing 1,1 and 1,2 insertion mechanisms. It highlights the requirements for these reactions, such as the necessity of cisoidal ligands and the unaltered oxidation states of metal centers. The text also outlines general features, types, and mechanisms associated with these reactions, supported by references for further study.