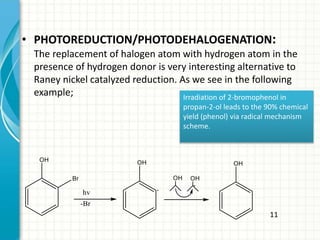
The document discusses photoreduction, which is the chemical reduction influenced by light energy. It specifically discusses photoreduction of carbonyl compounds like ketones, where ketones can be reduced by hydrogen atom donors. Nitro compounds also undergo photoreduction with hydrogen donors, otherwise photofragmentation occurs. Aromatic compounds like benzene and naphthalene can be photoreduced through electron transfer from amines. Photoreduction and photodehalogenation involve replacing halogen atoms with hydrogen. Photoreduction is also used in polymerization by producing initiators through reduction that react with monomers.