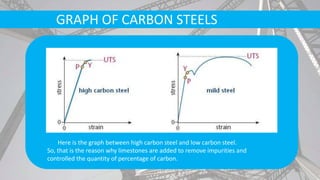
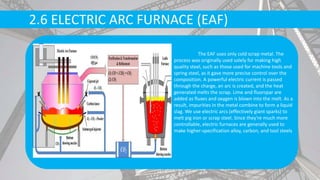
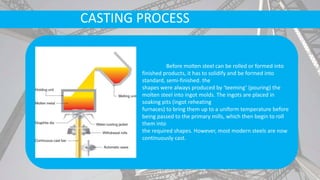
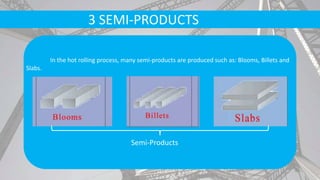
The document discusses the process of making steel, including the raw materials, production processes, and applications. Key points:
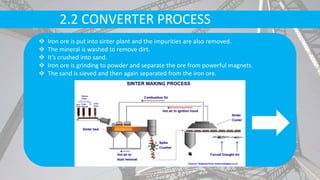
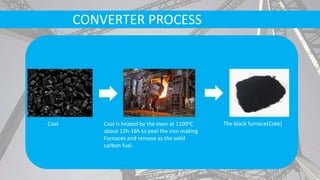
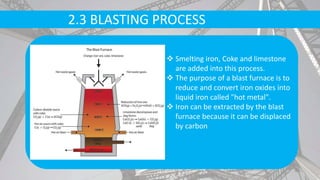
- Raw materials include iron ore, limestone, coke, and scrap metal. Iron ore is purified and melted in blast furnaces with coke and limestone.
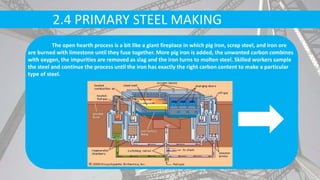
- Primary production methods are basic oxygen furnace (BOF) and electric arc furnace (EAF), which further refine molten iron into steel.
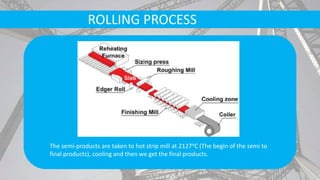
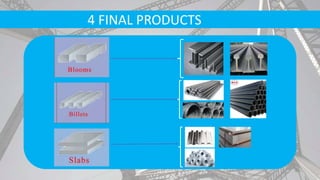
- Secondary processes like degassing and temperature control produce semi-finished and finished steel products.
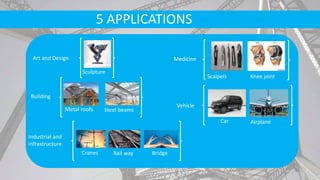
- Steel products have many applications in infrastructure, vehicles, machinery, and more due to their strength and cost effectiveness.