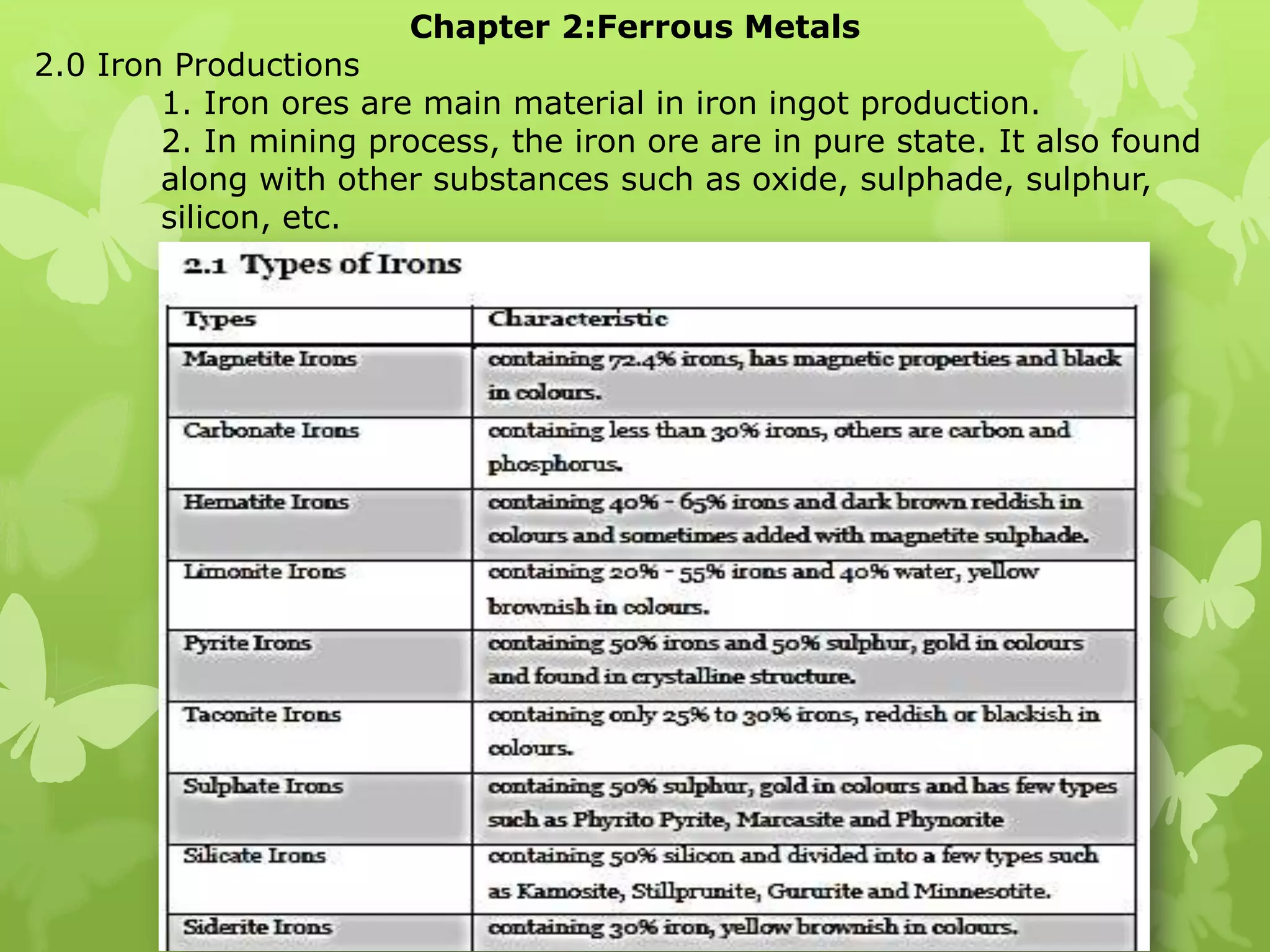
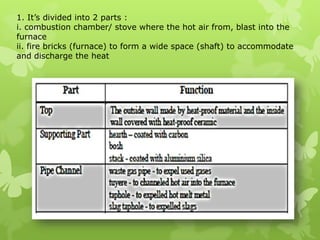
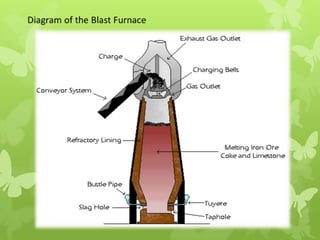
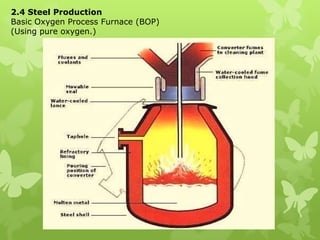
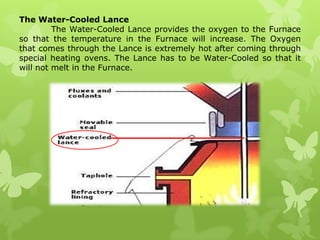
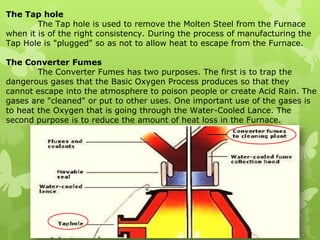
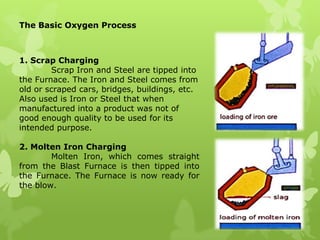
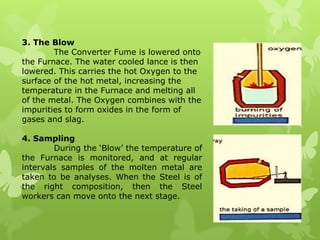
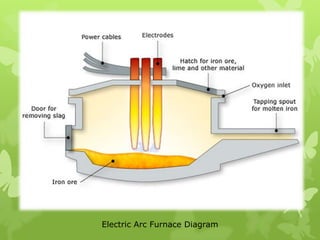
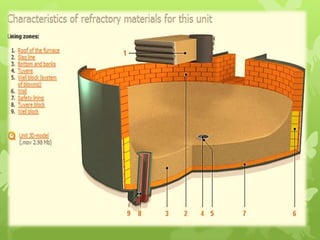
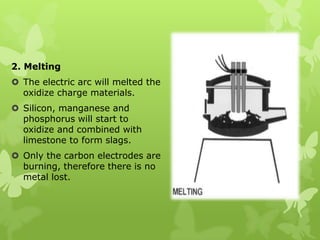
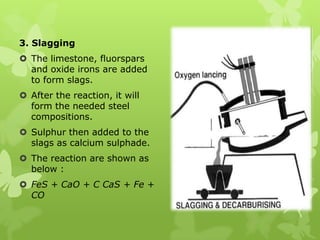
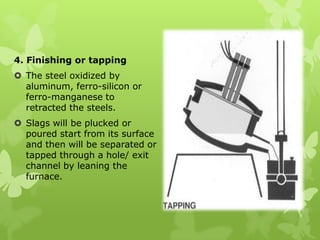
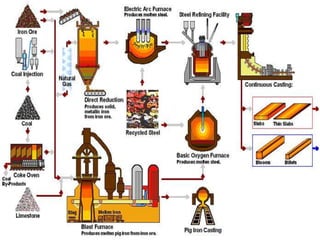
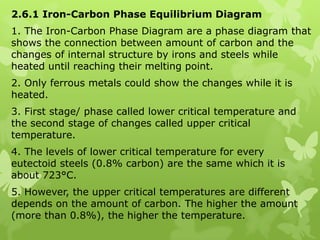
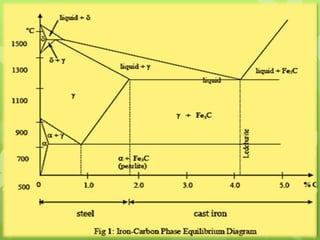
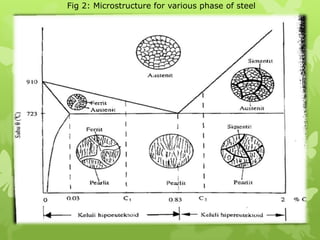
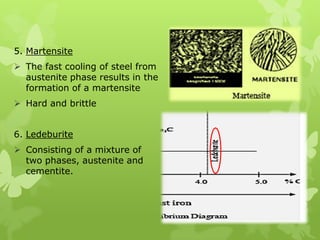
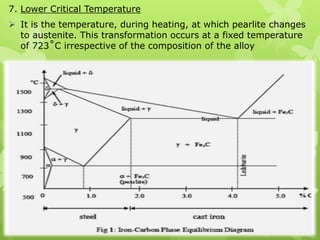
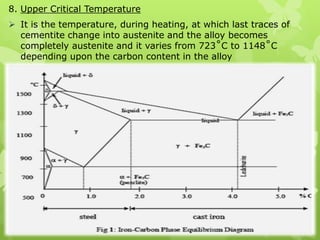
The document summarizes information about ferrous metals and steel production processes. It discusses the characteristics of iron ores and how the blast furnace process is used to produce iron from iron ores. The basic oxygen process and electric arc furnace processes are also summarized as methods for producing steel from iron. Key aspects of these steel production methods include using oxygen and electricity respectively to burn off impurities from iron and produce steel alloys with the desired carbon content. Phase diagrams are also discussed as a way to illustrate the changes in iron and steel structures at different carbon levels and temperatures.