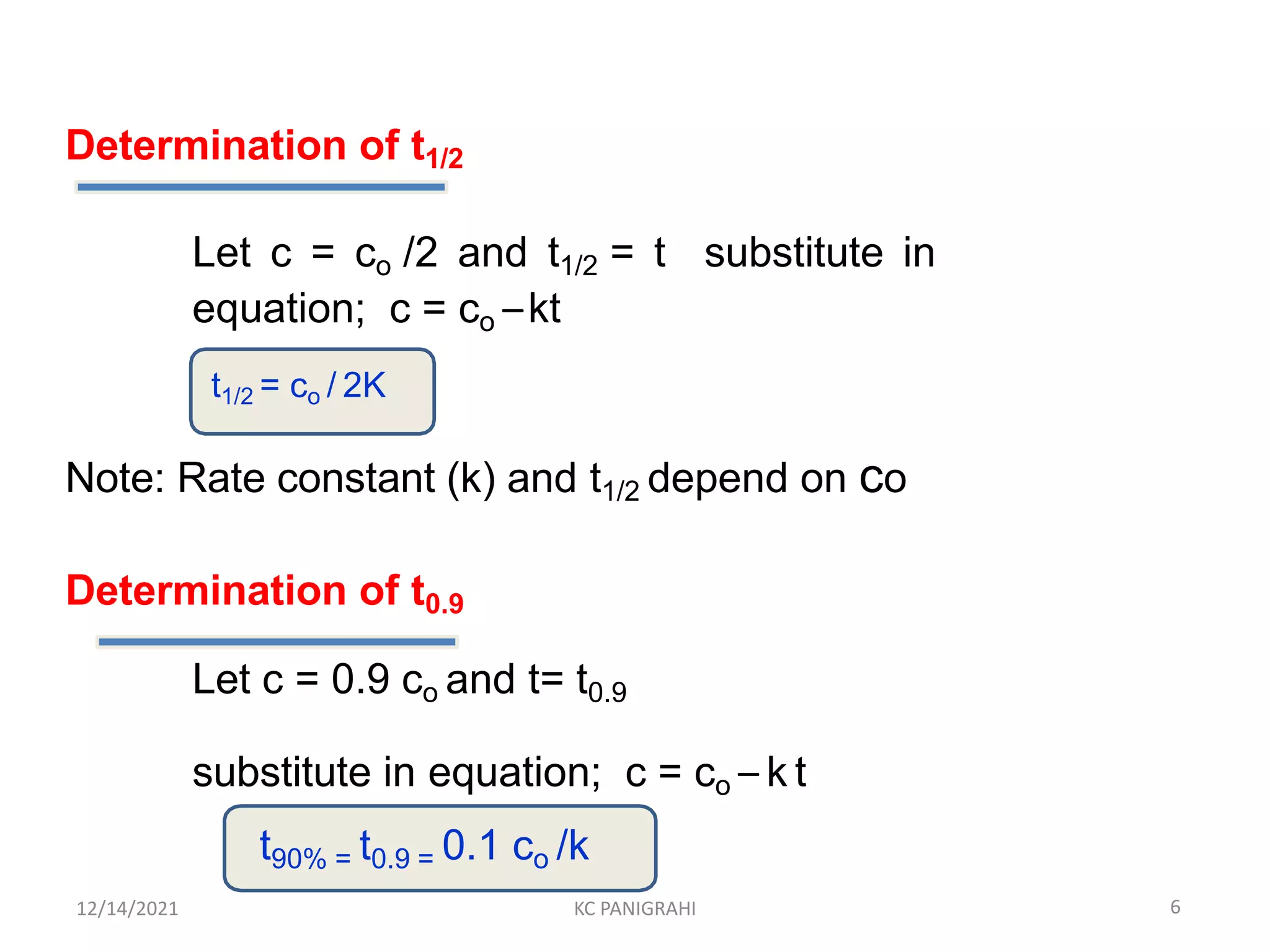
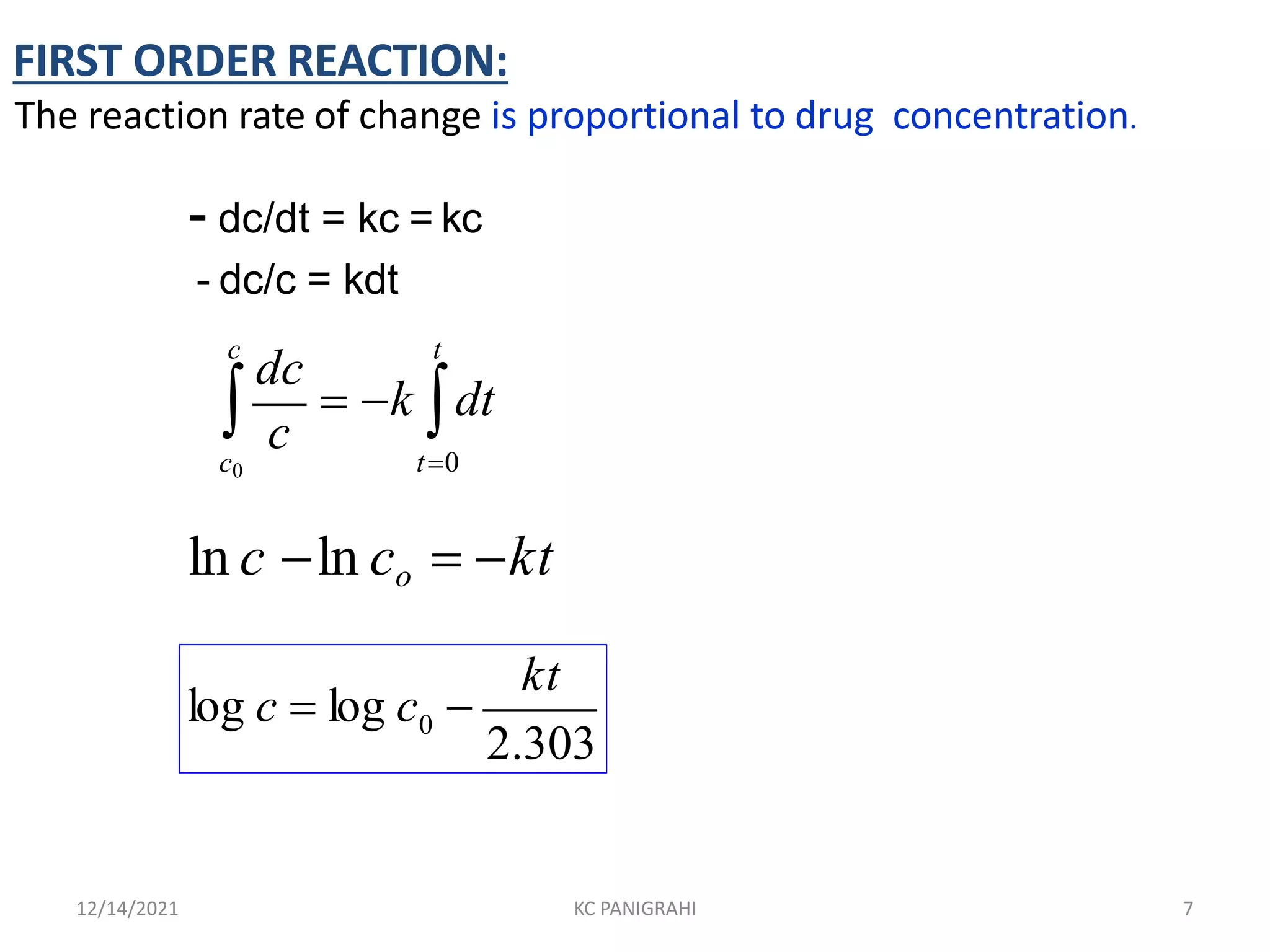
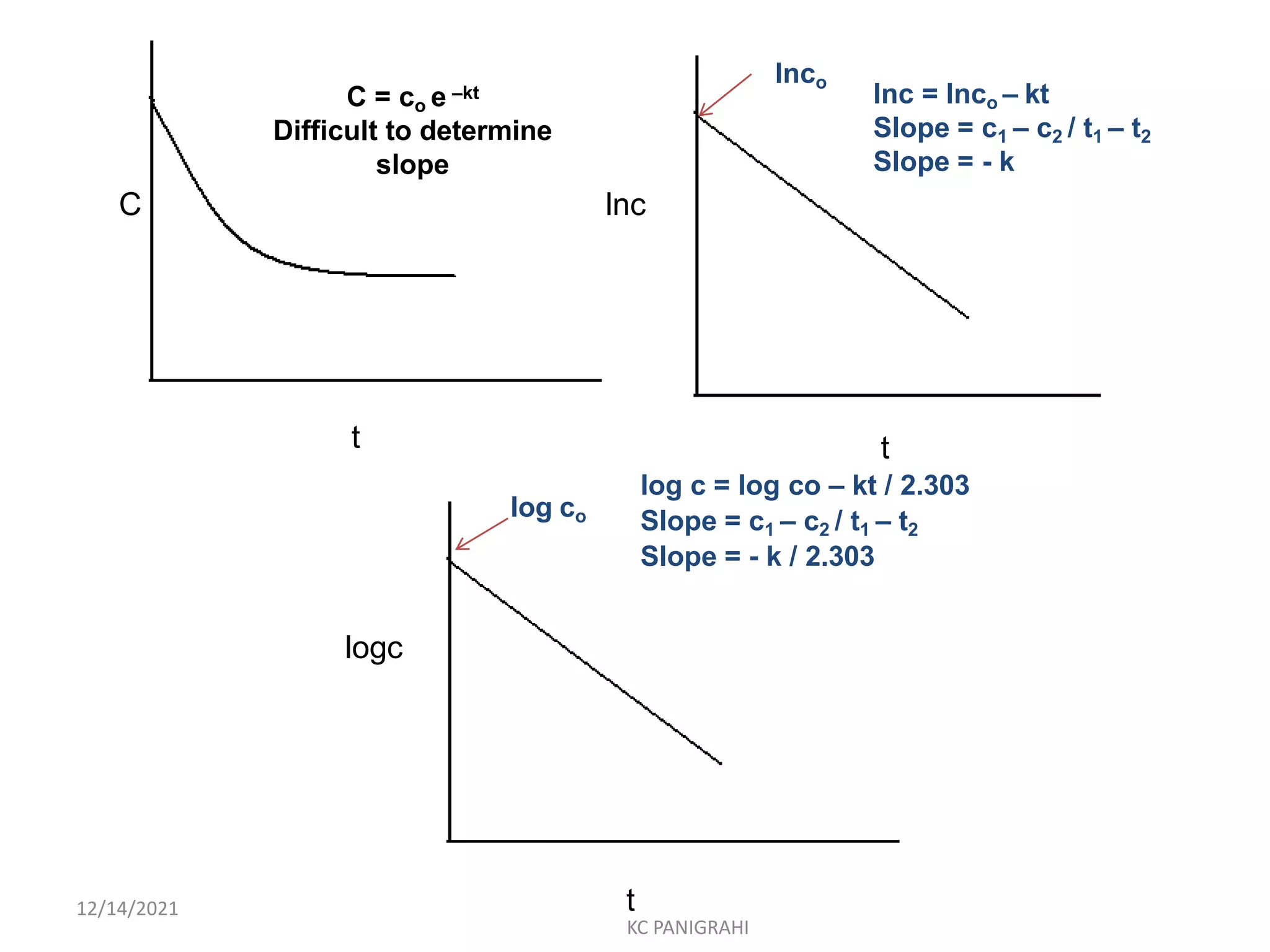
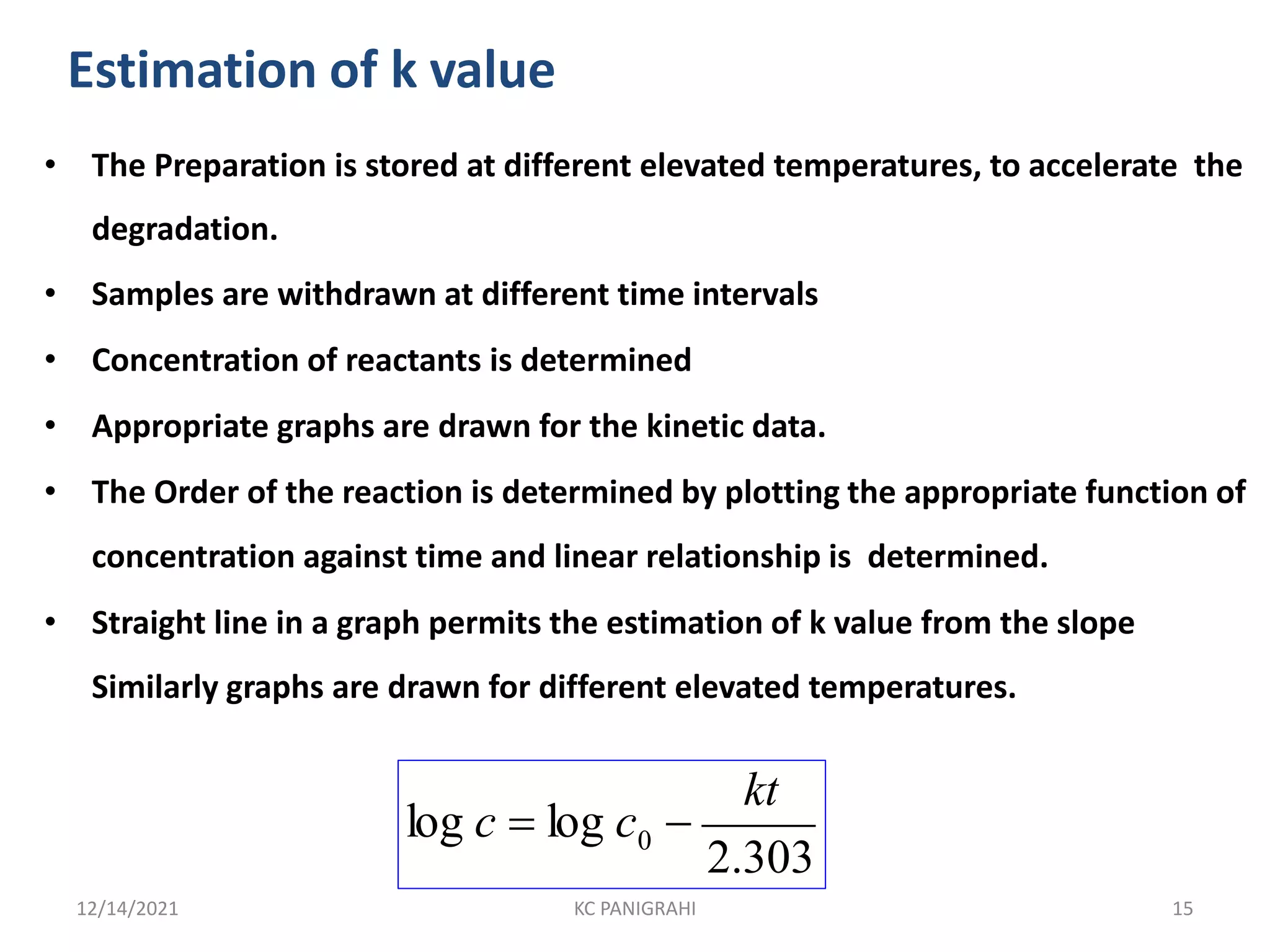
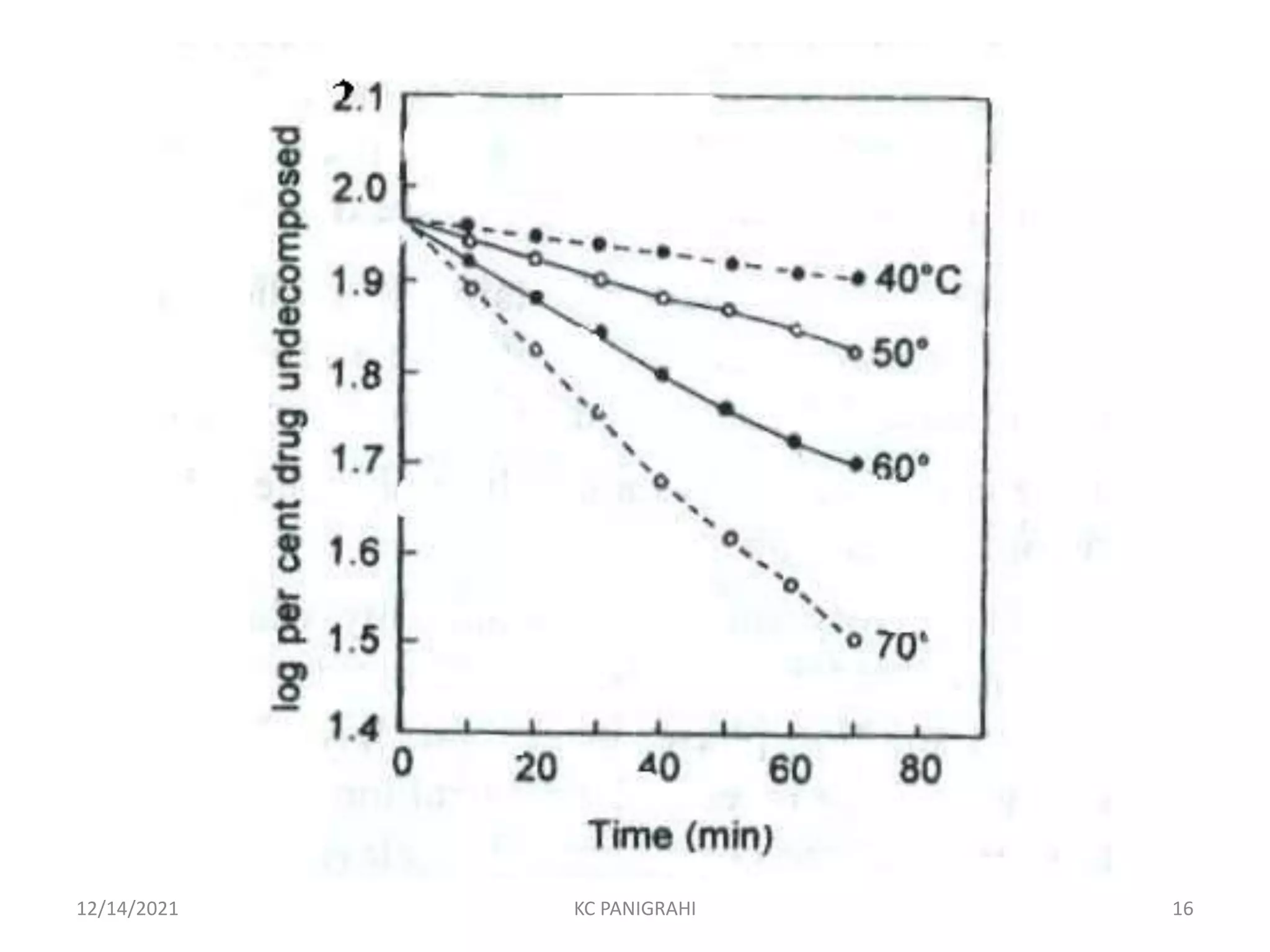
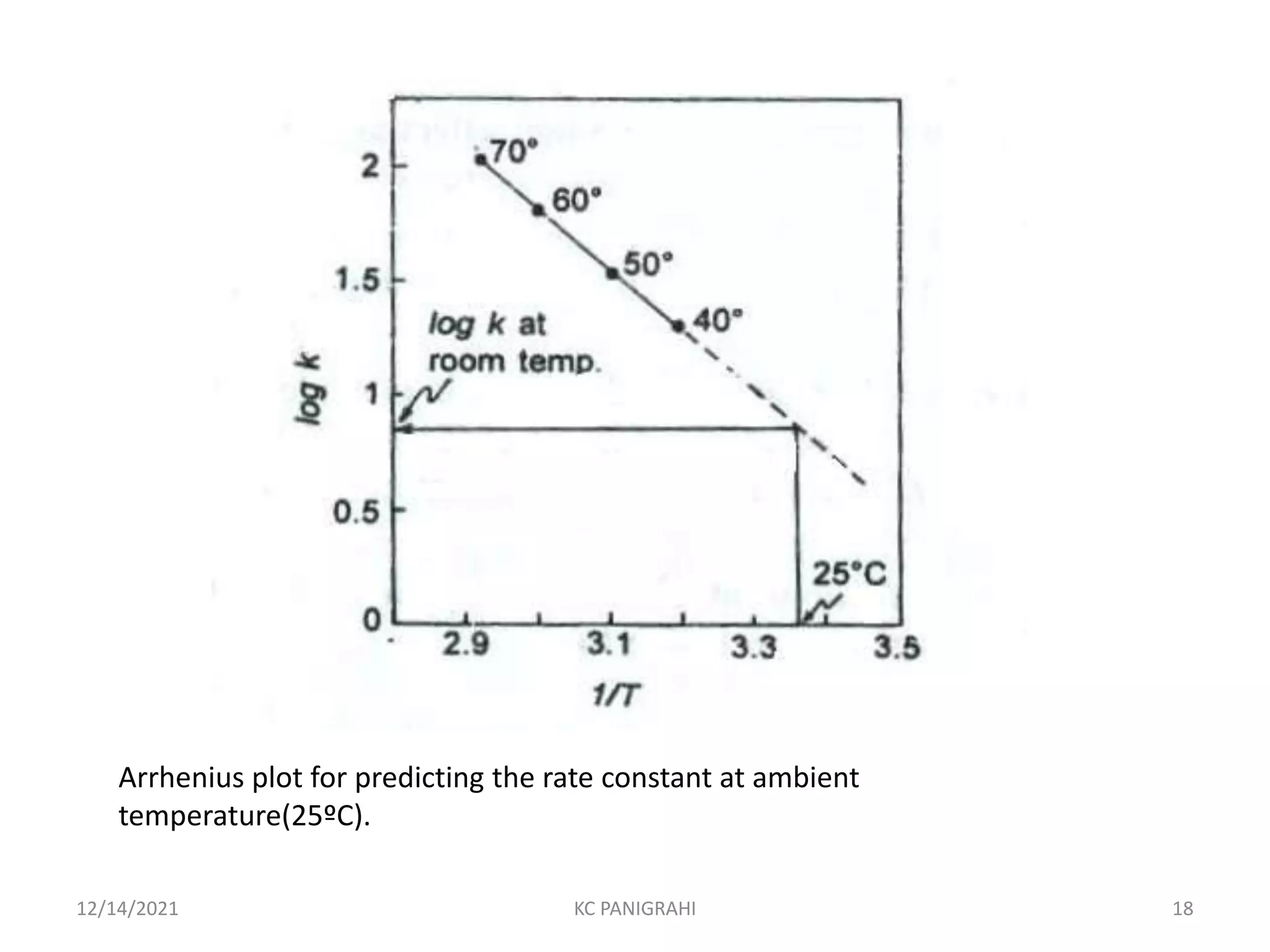
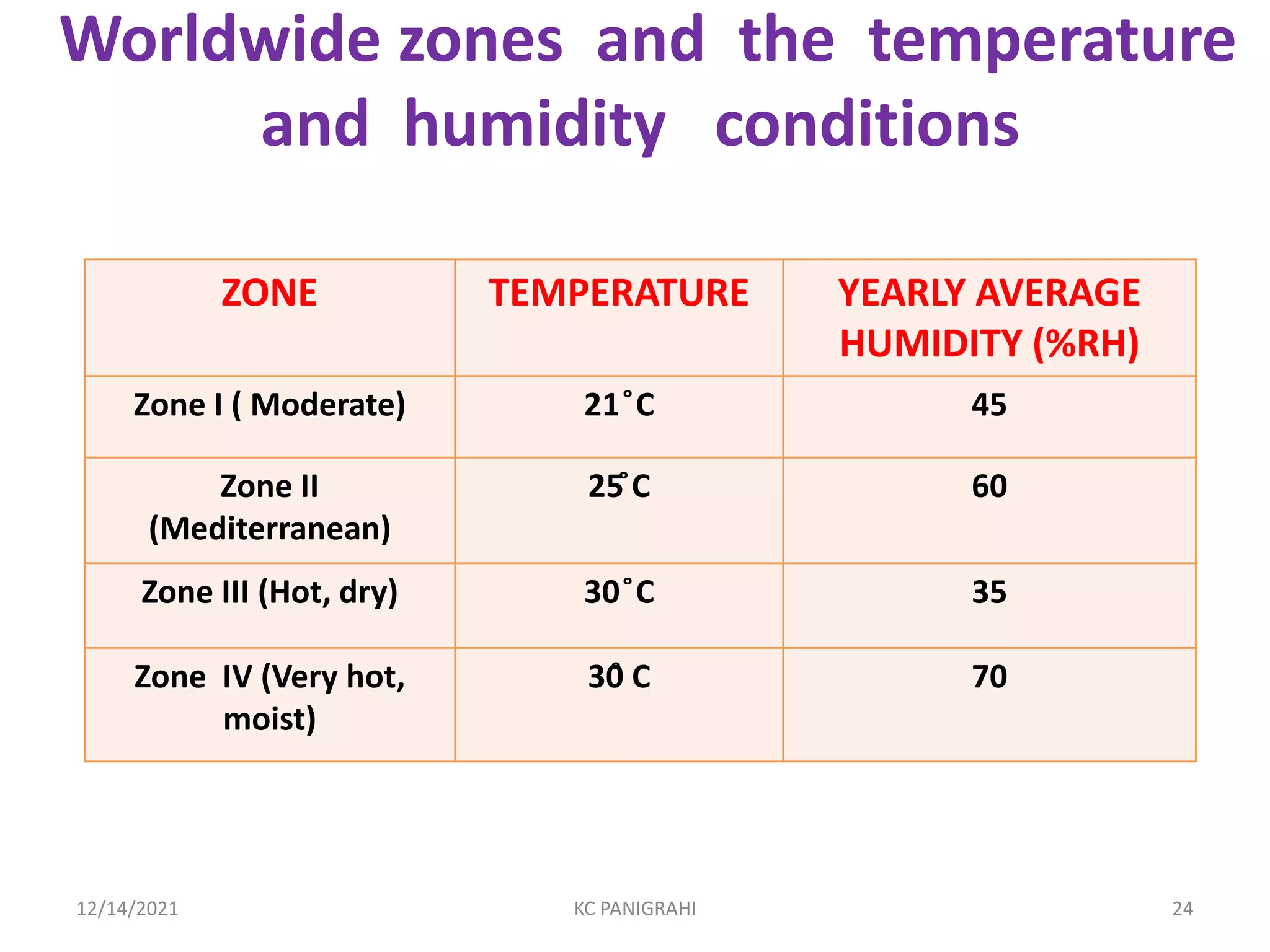
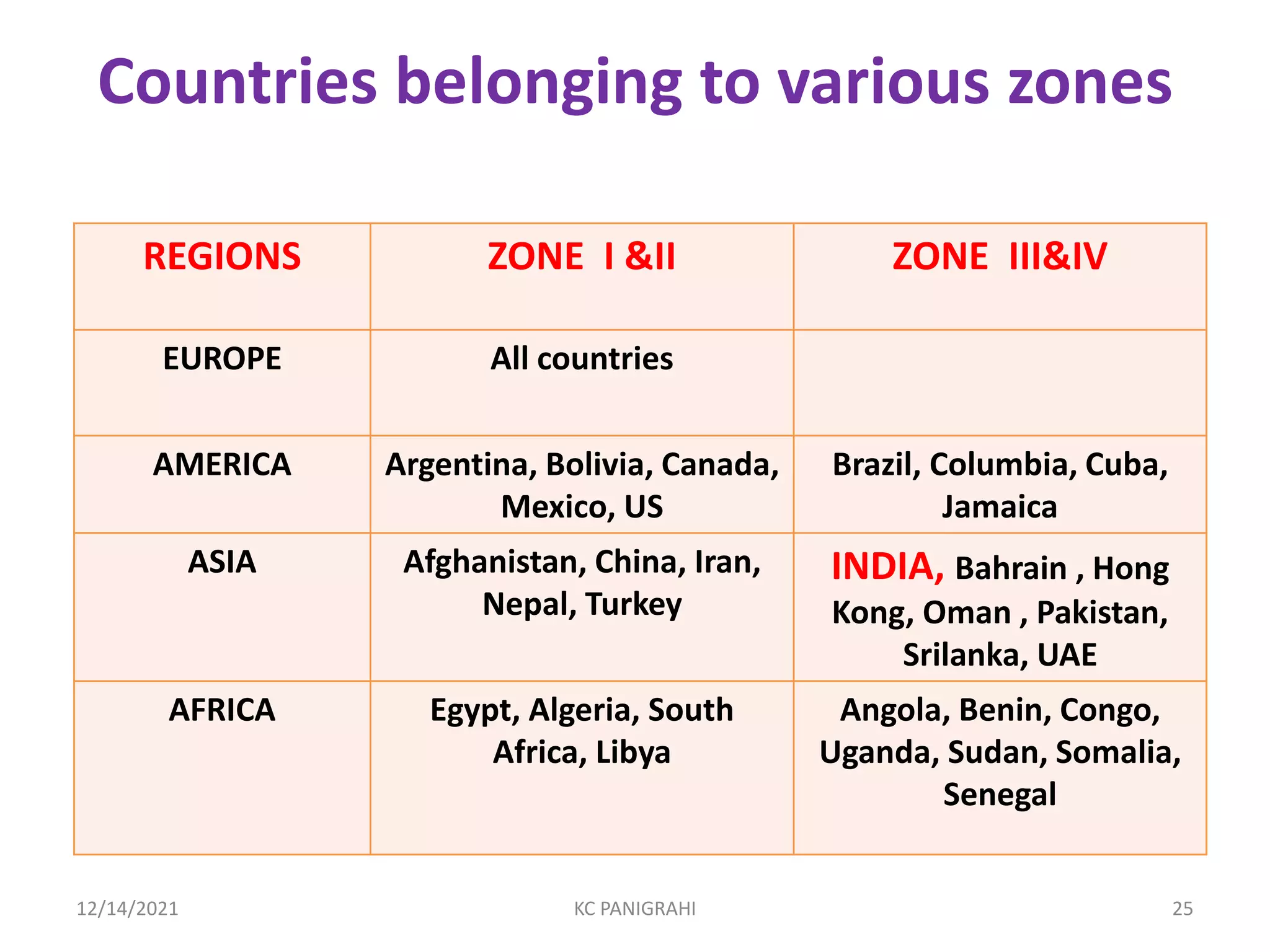
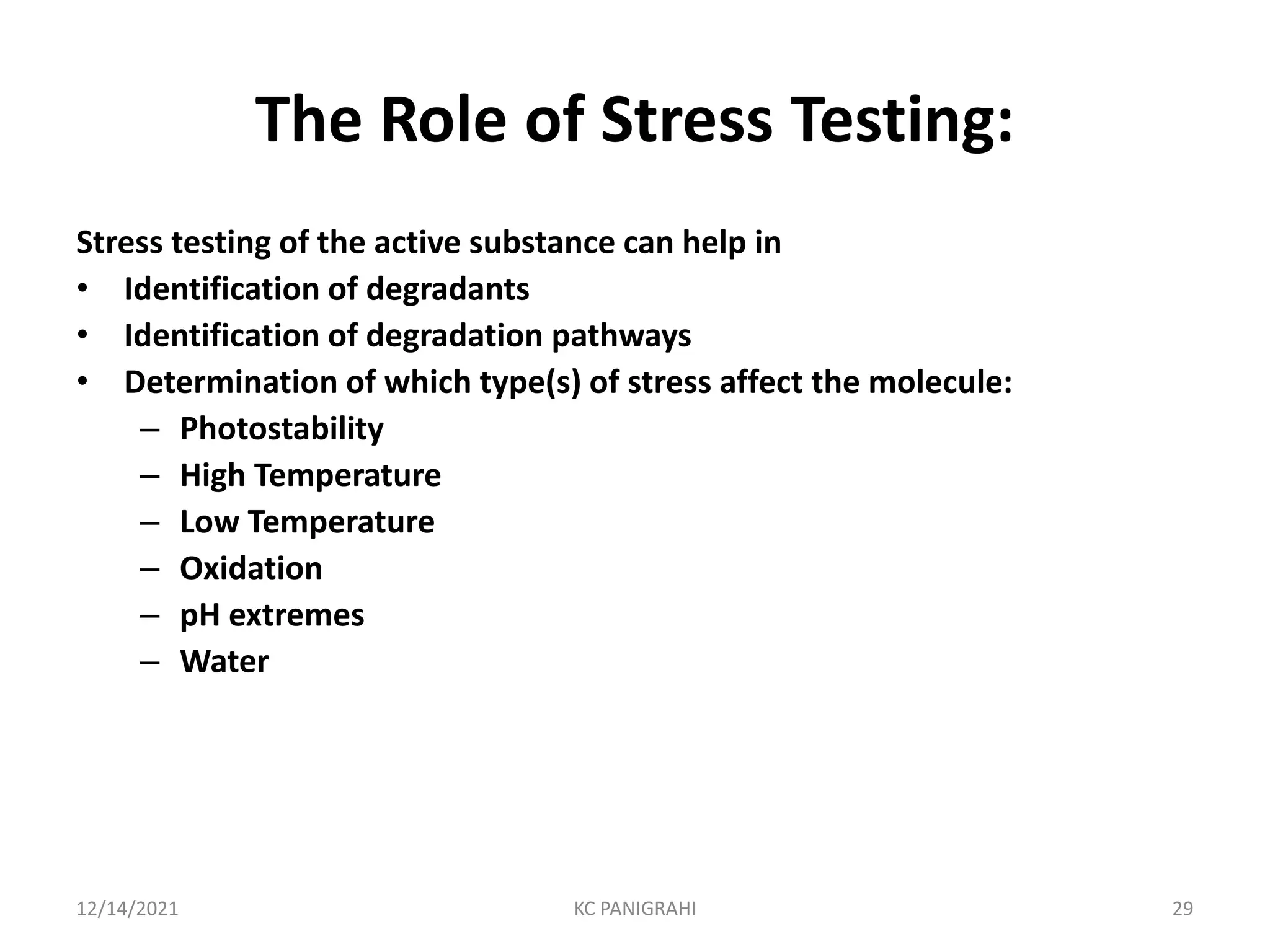
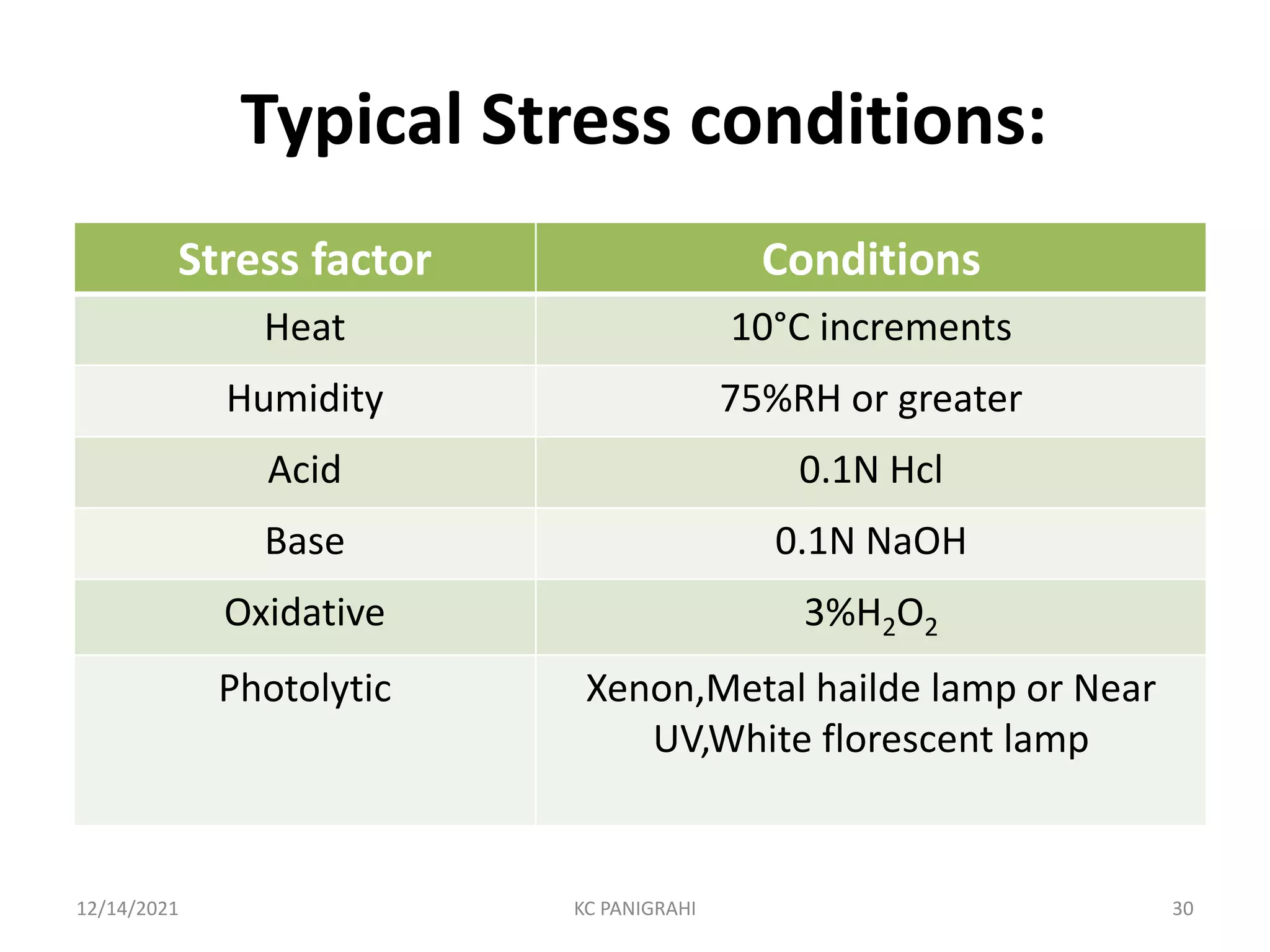
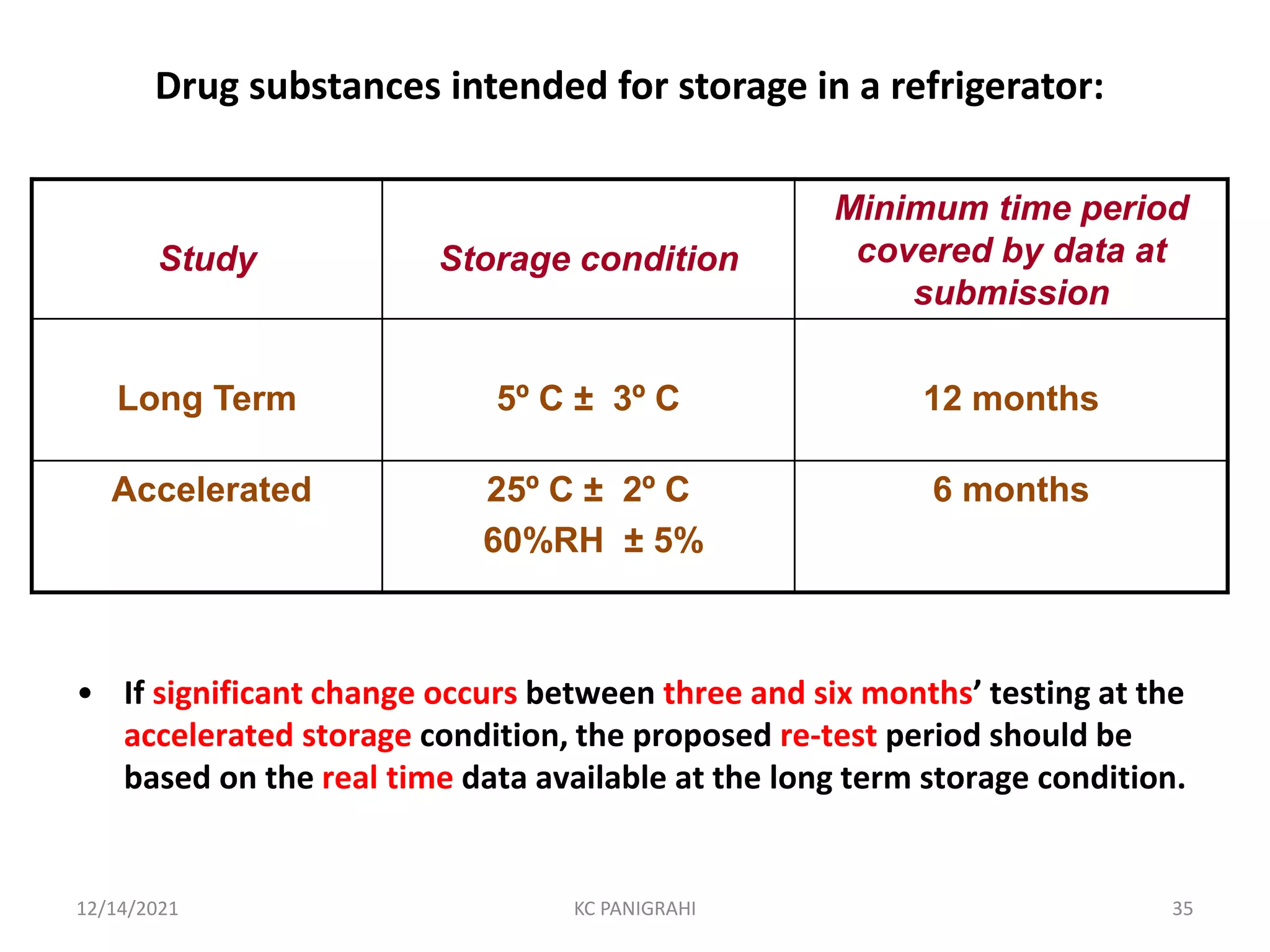
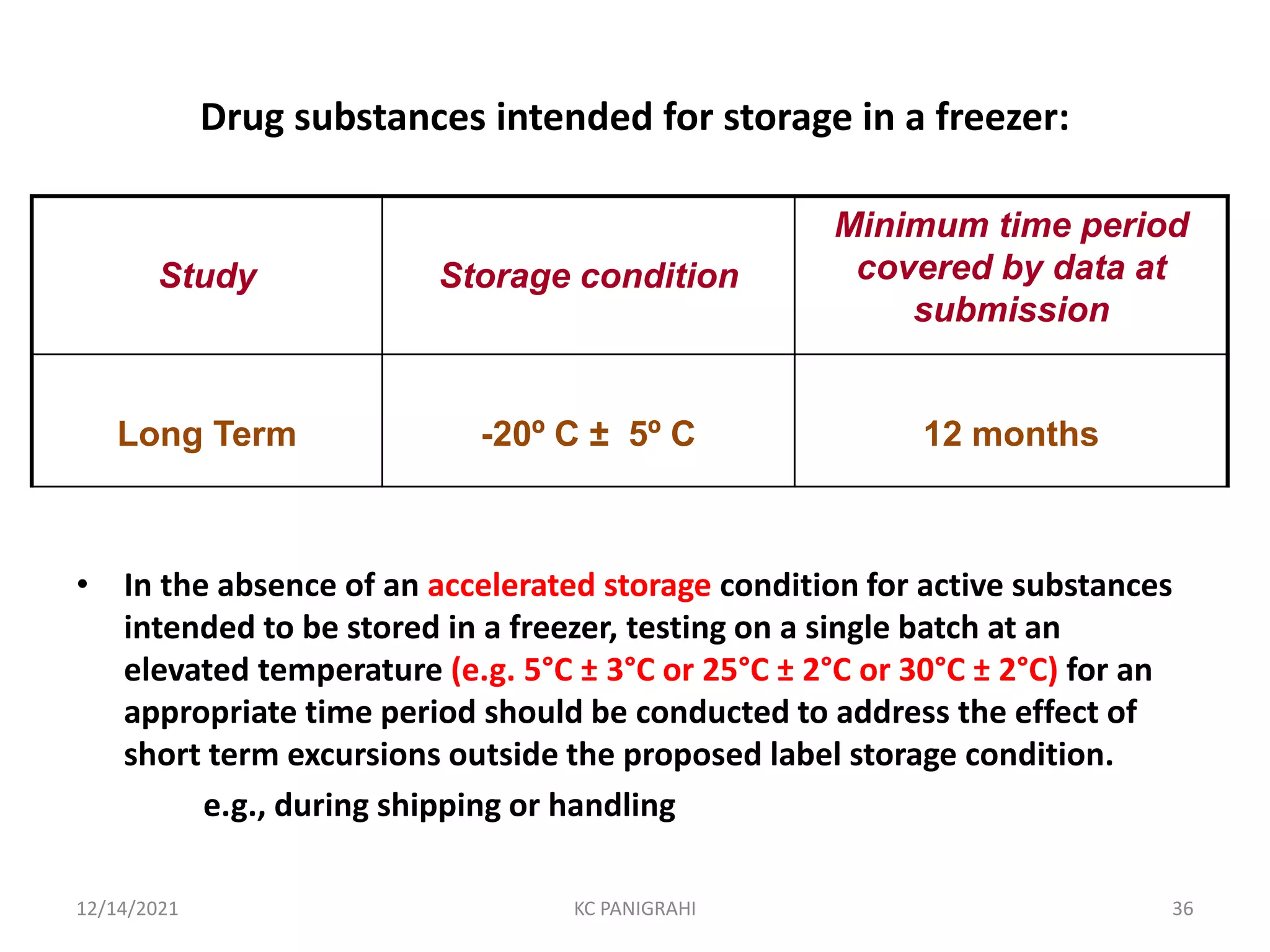
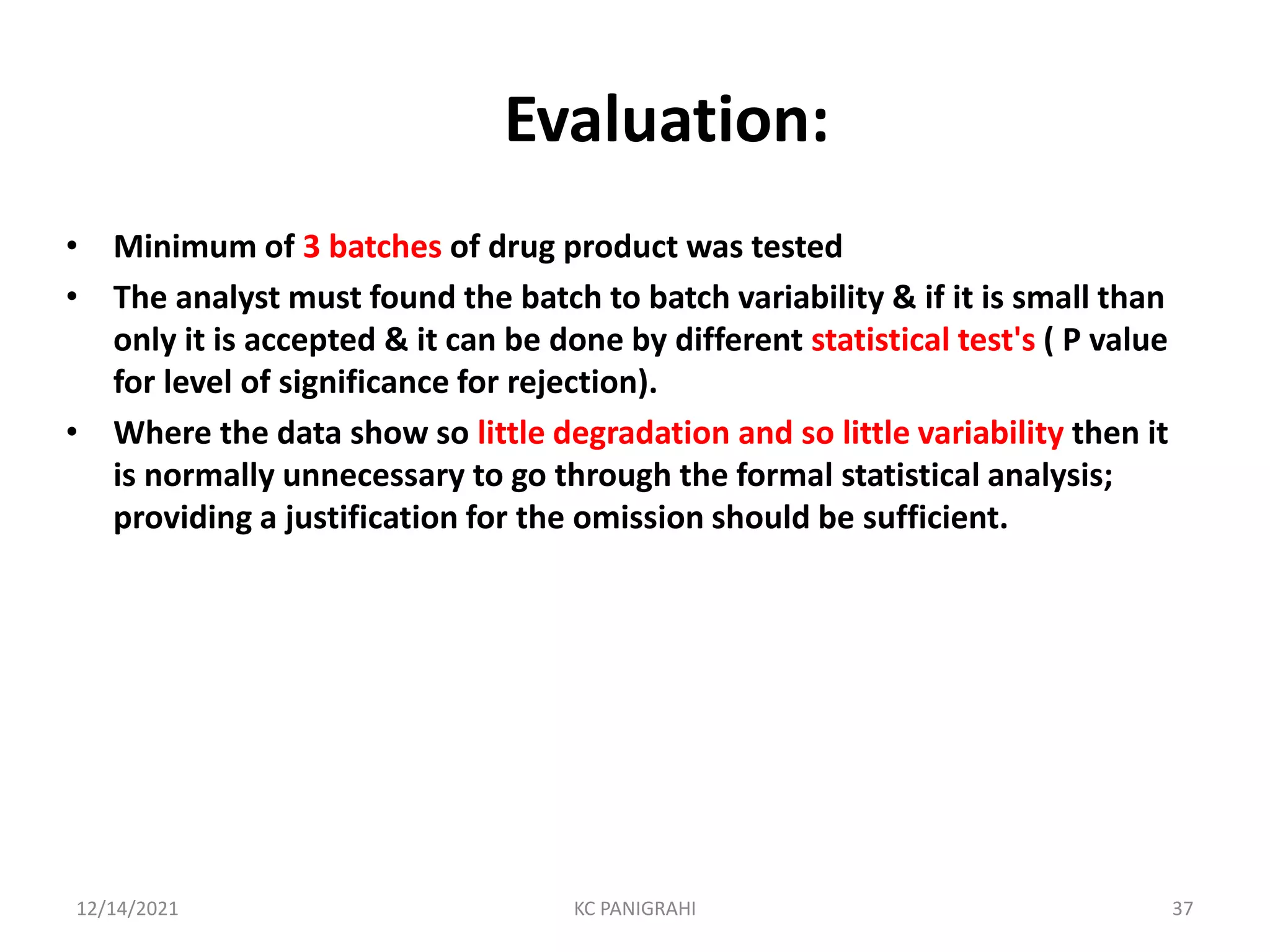
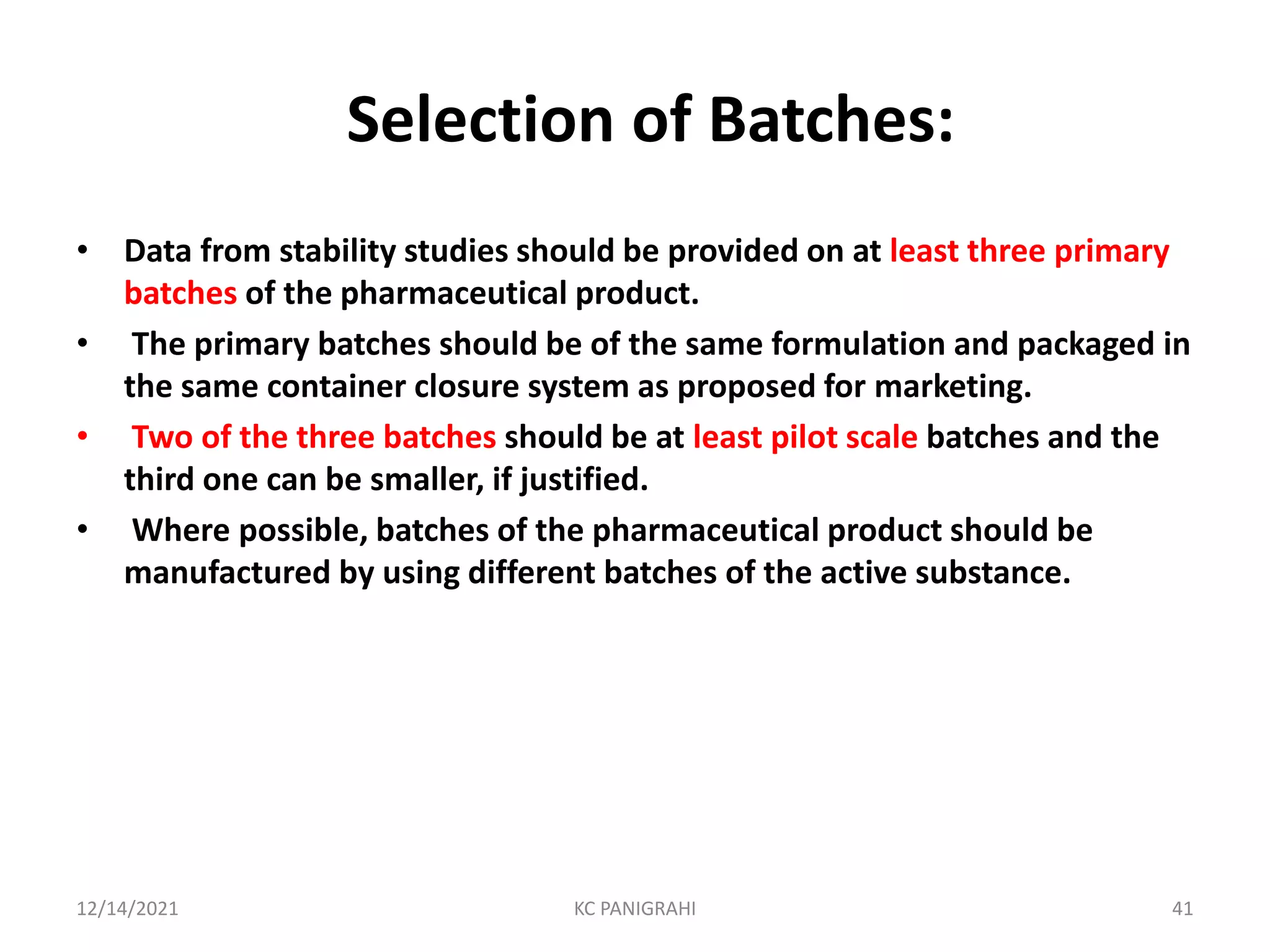
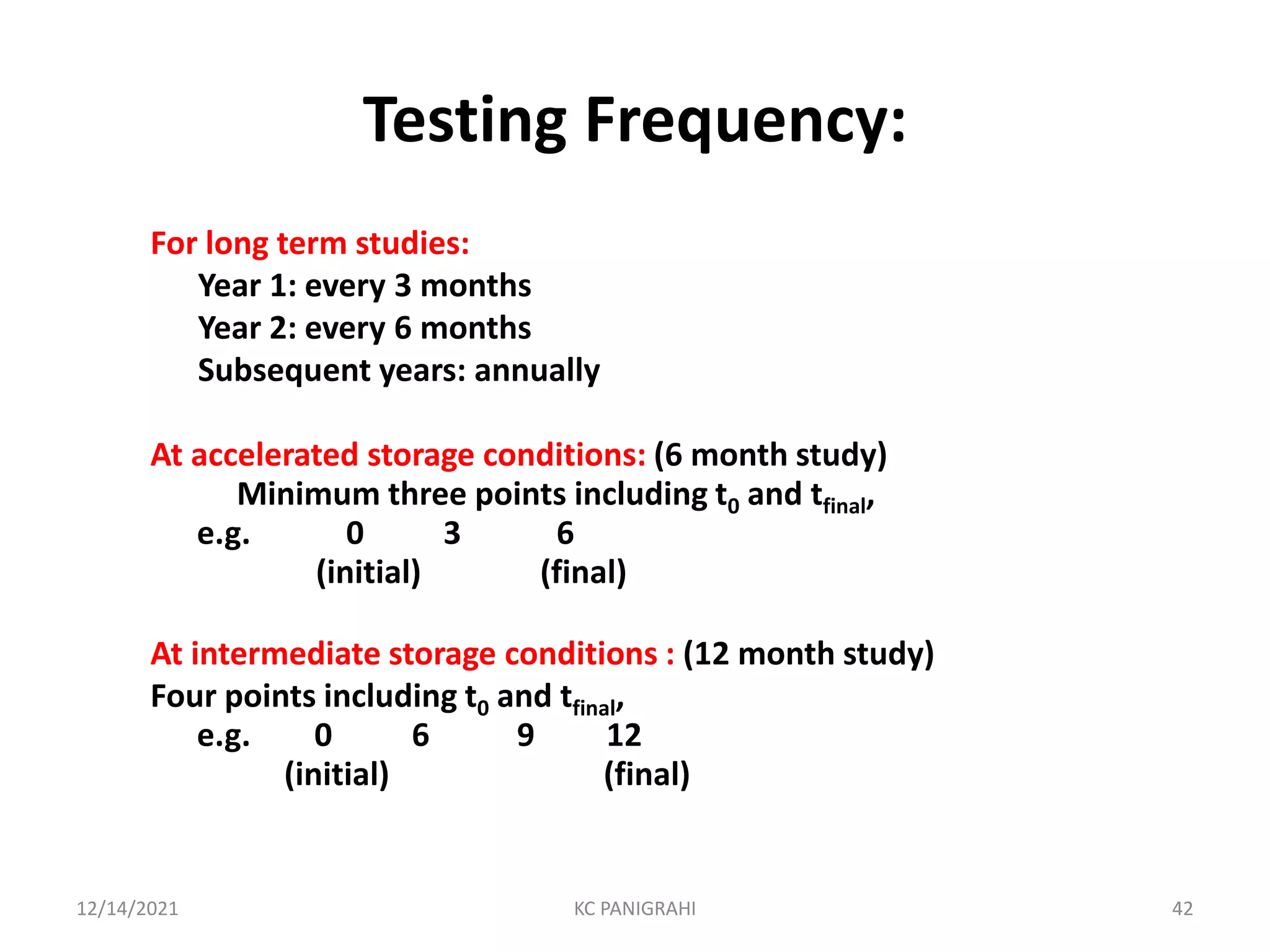
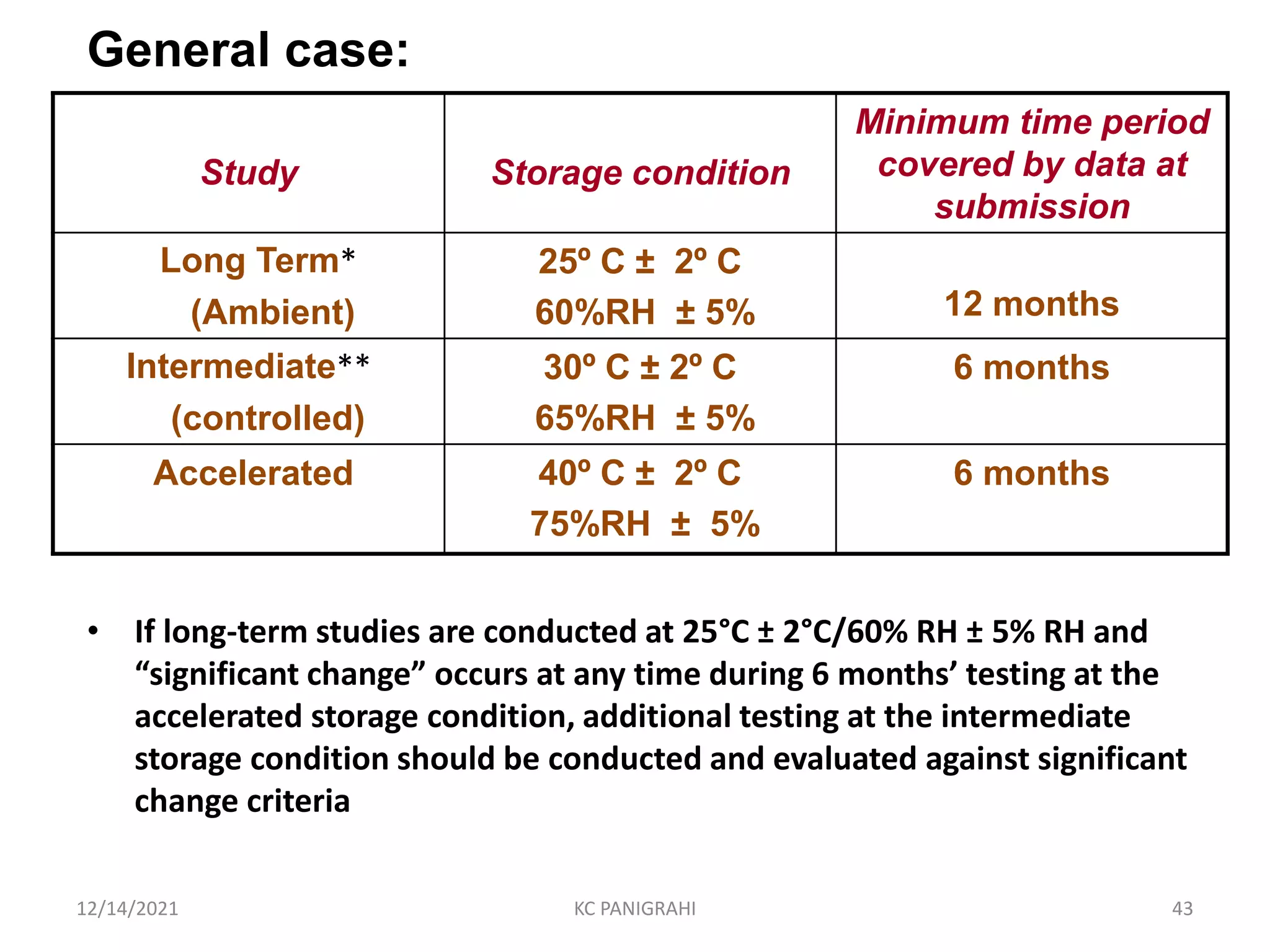
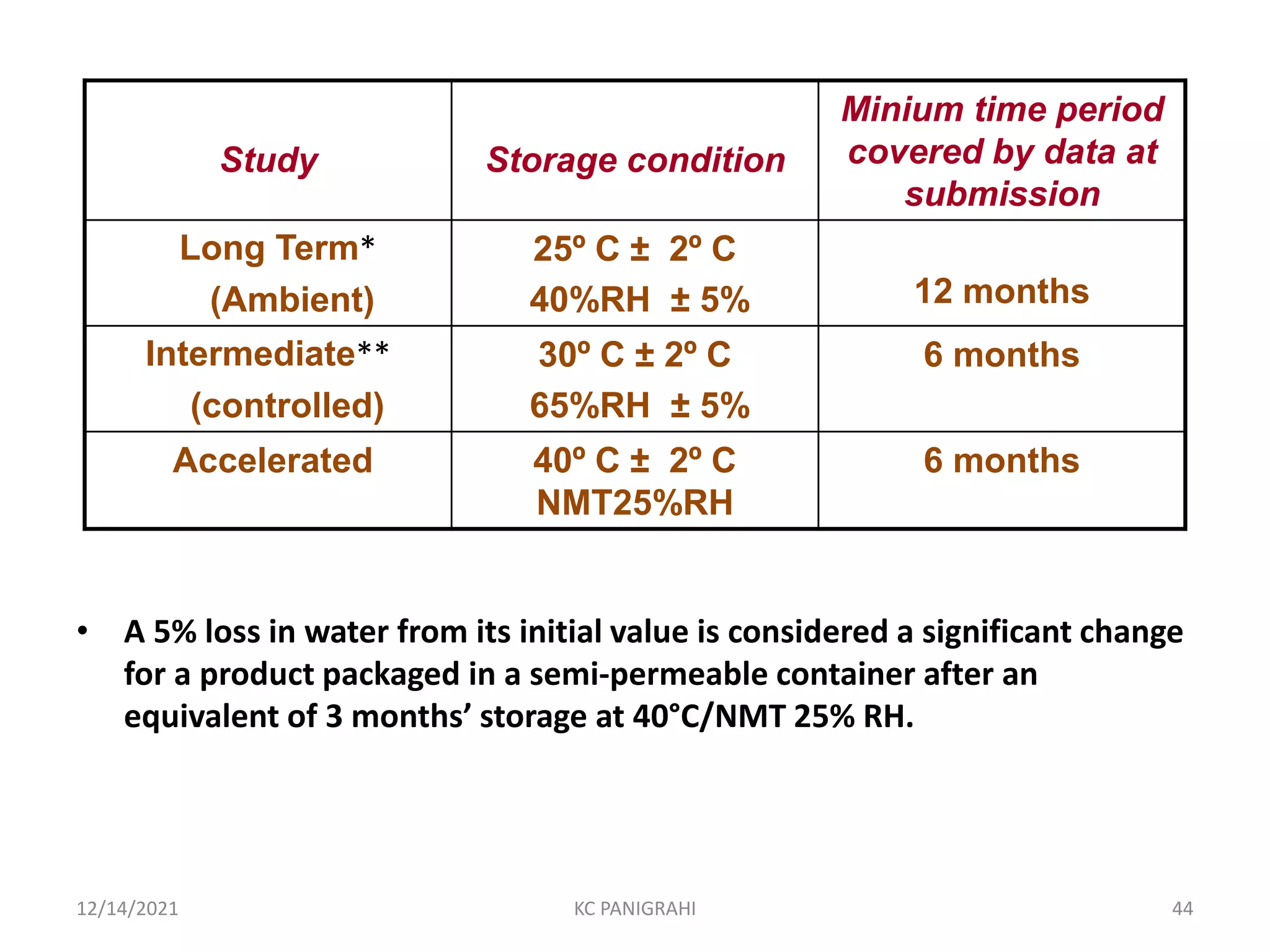
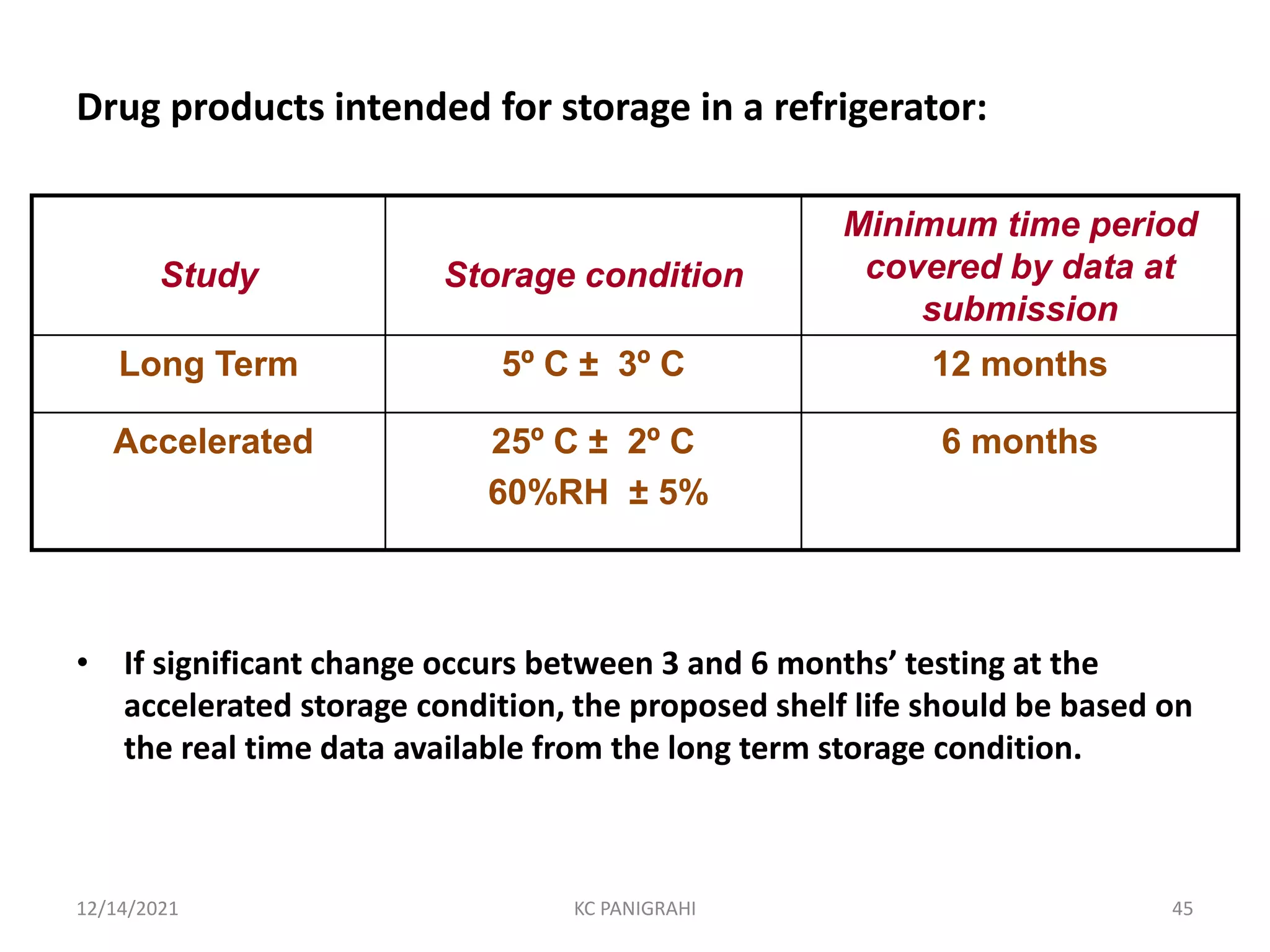
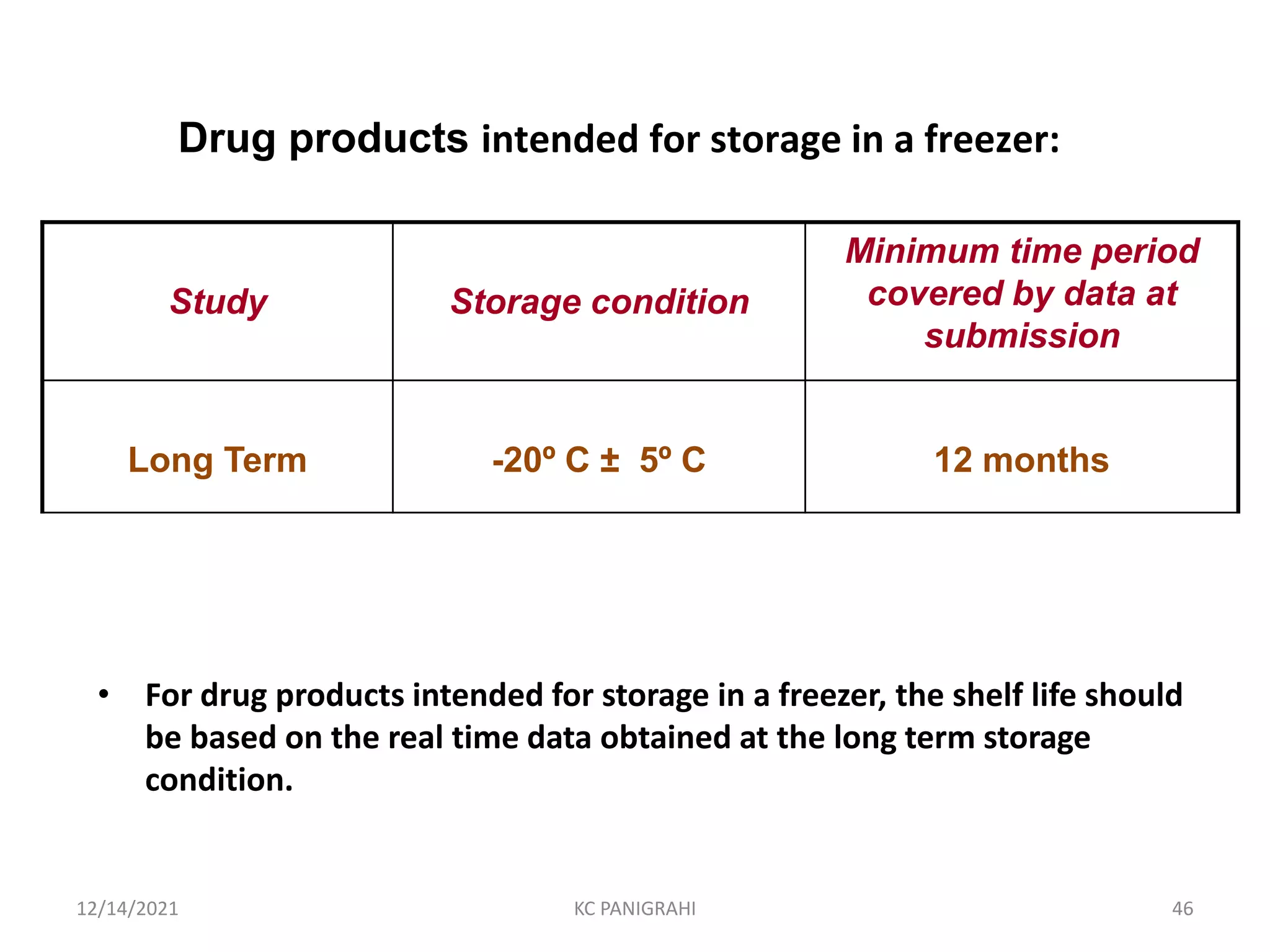
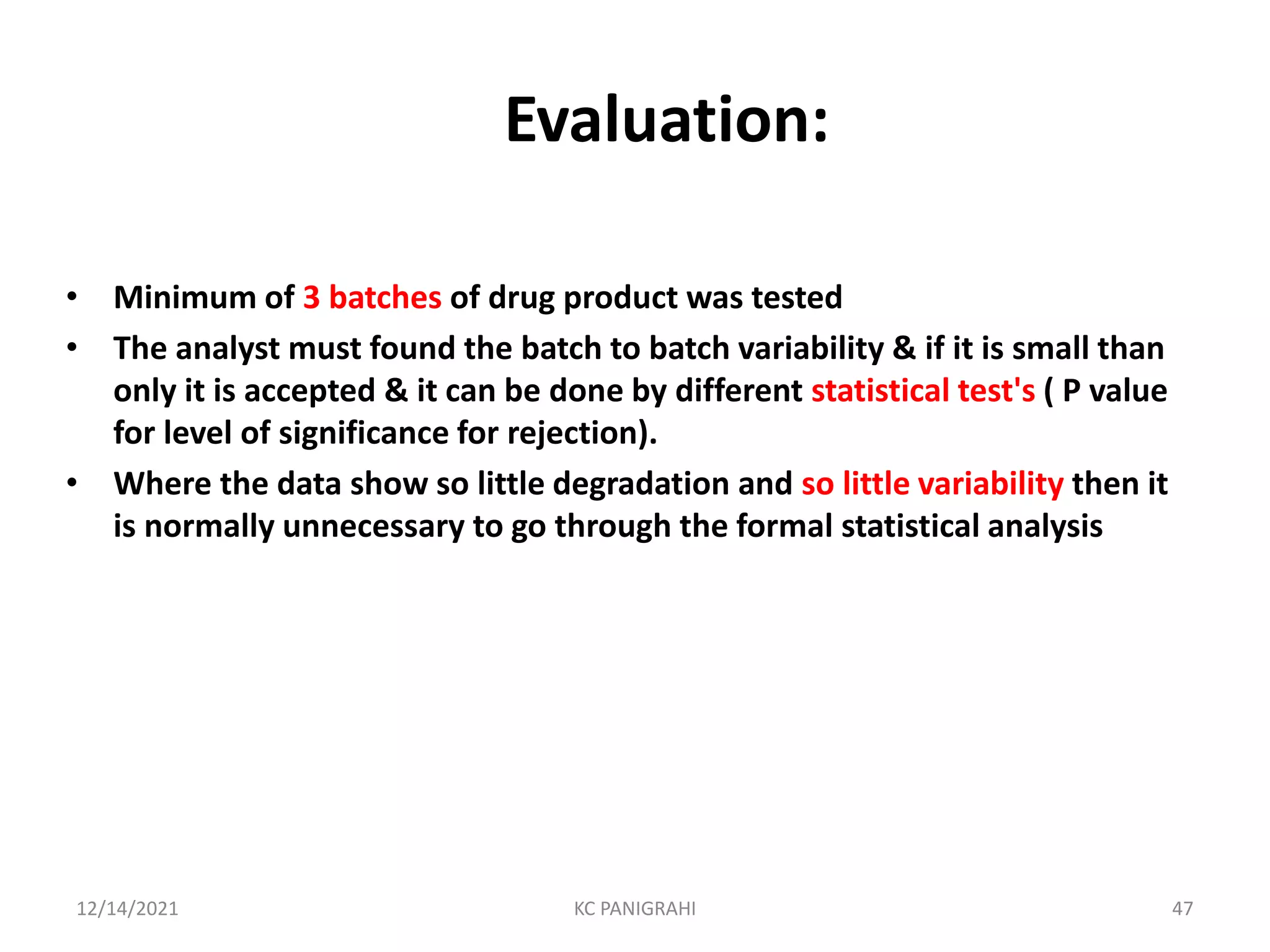
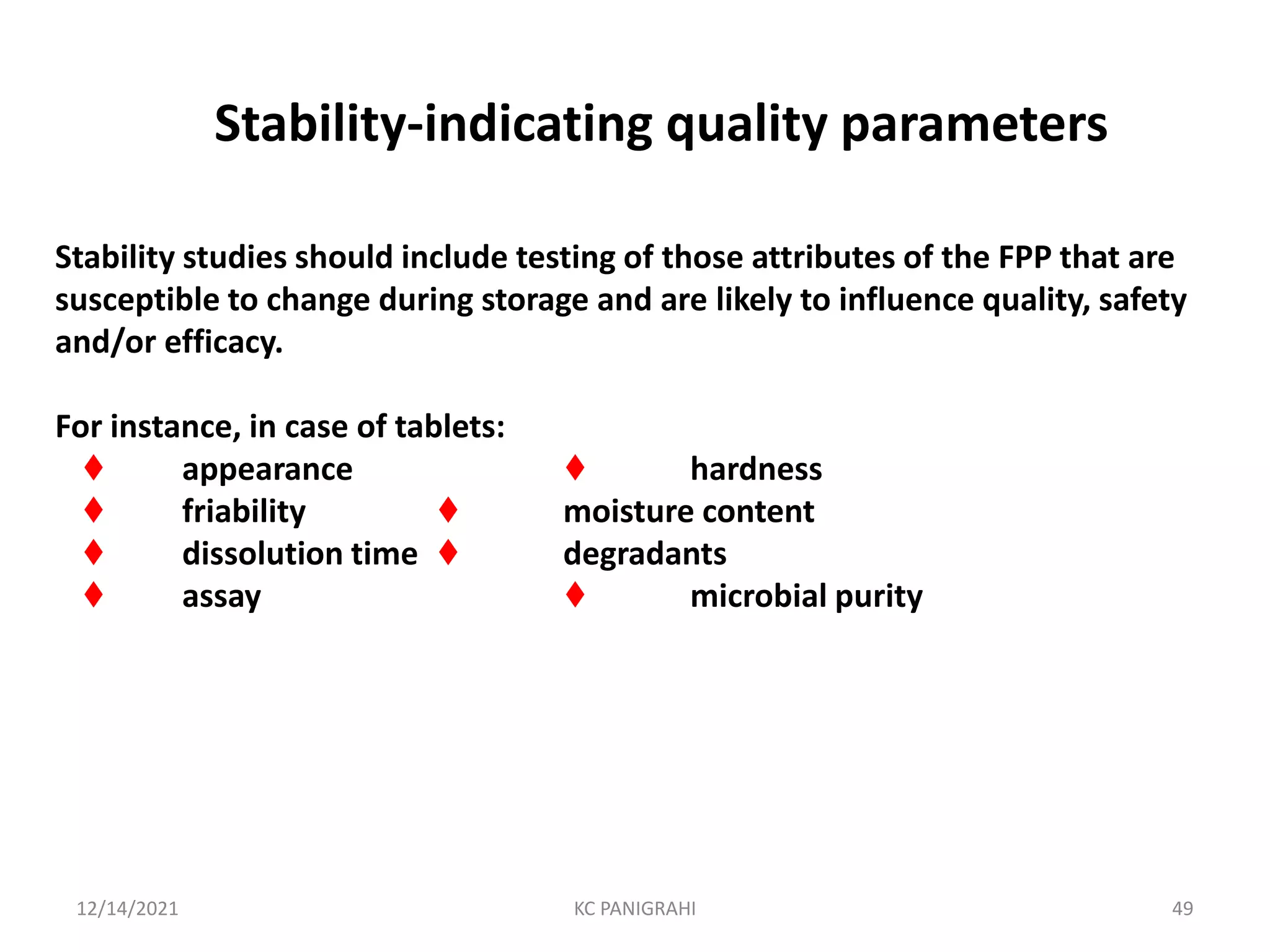
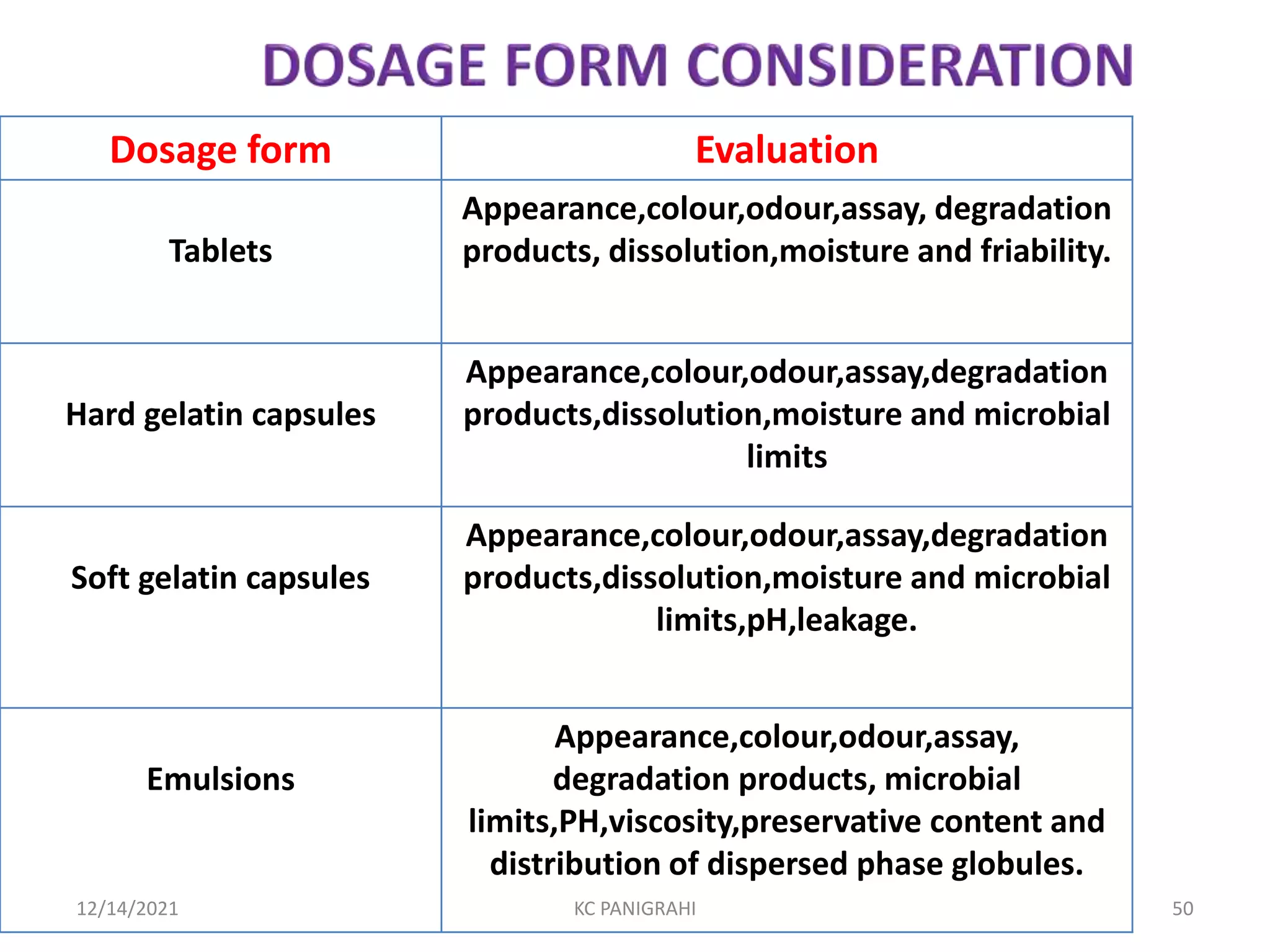
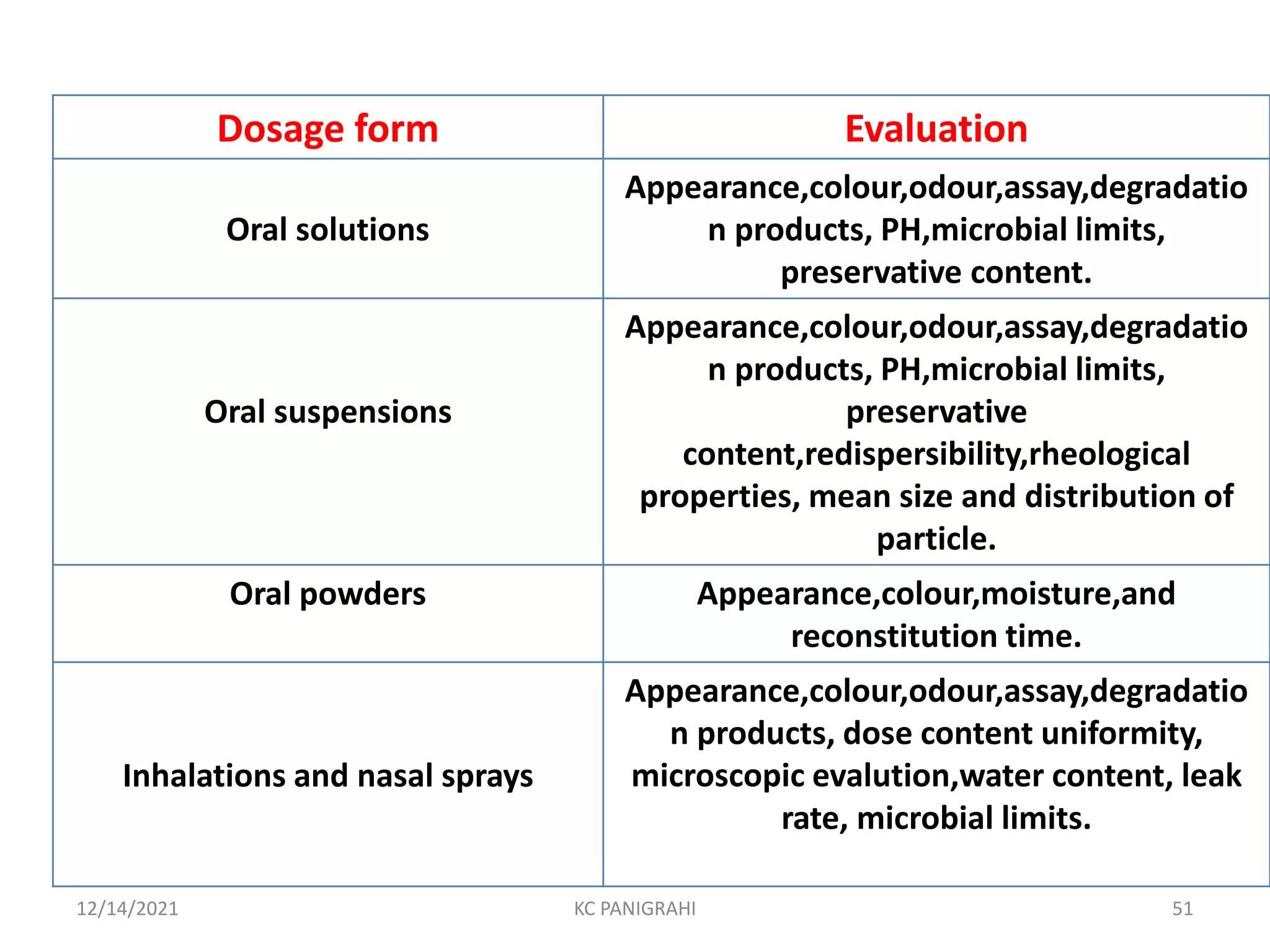
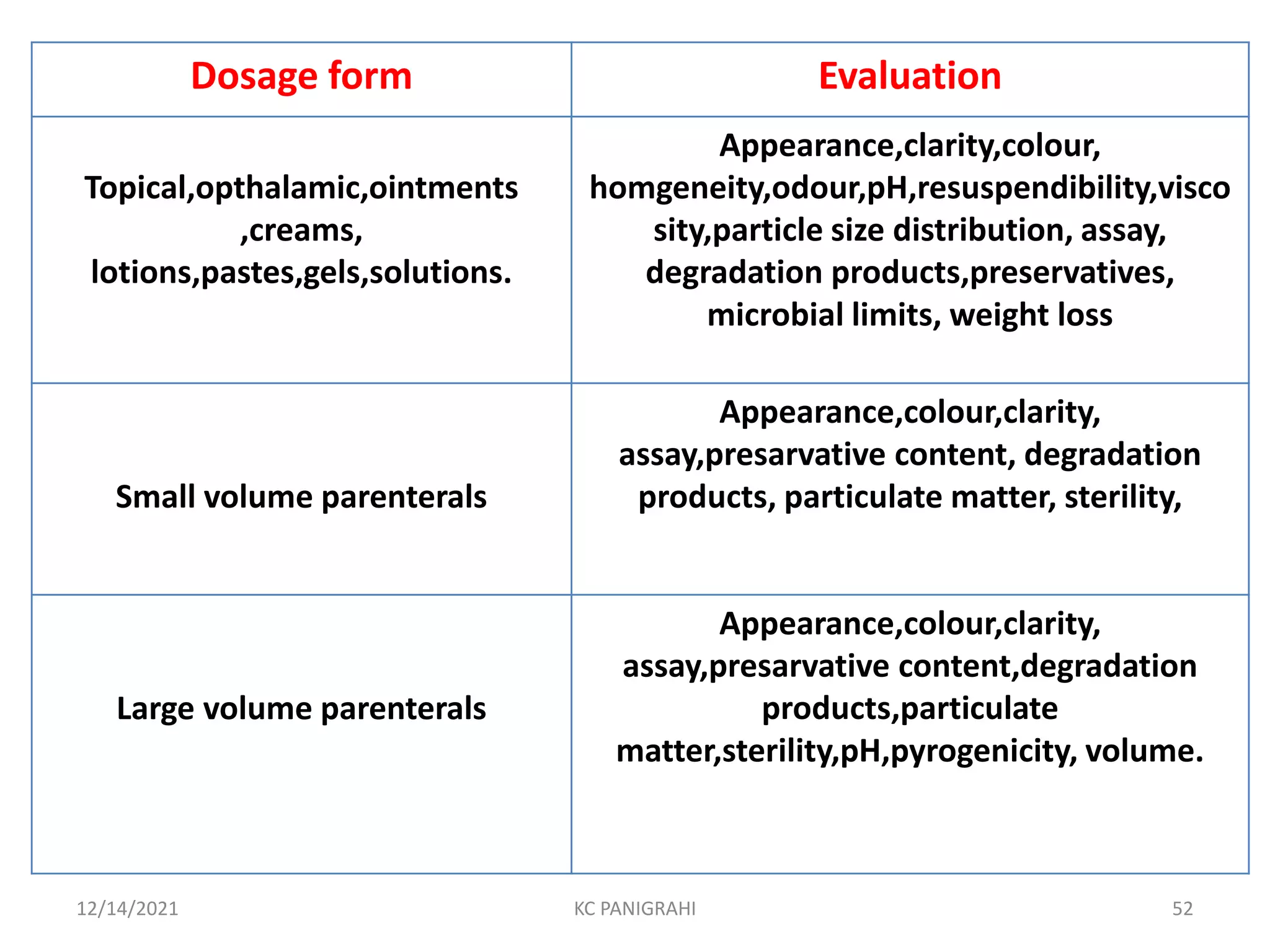
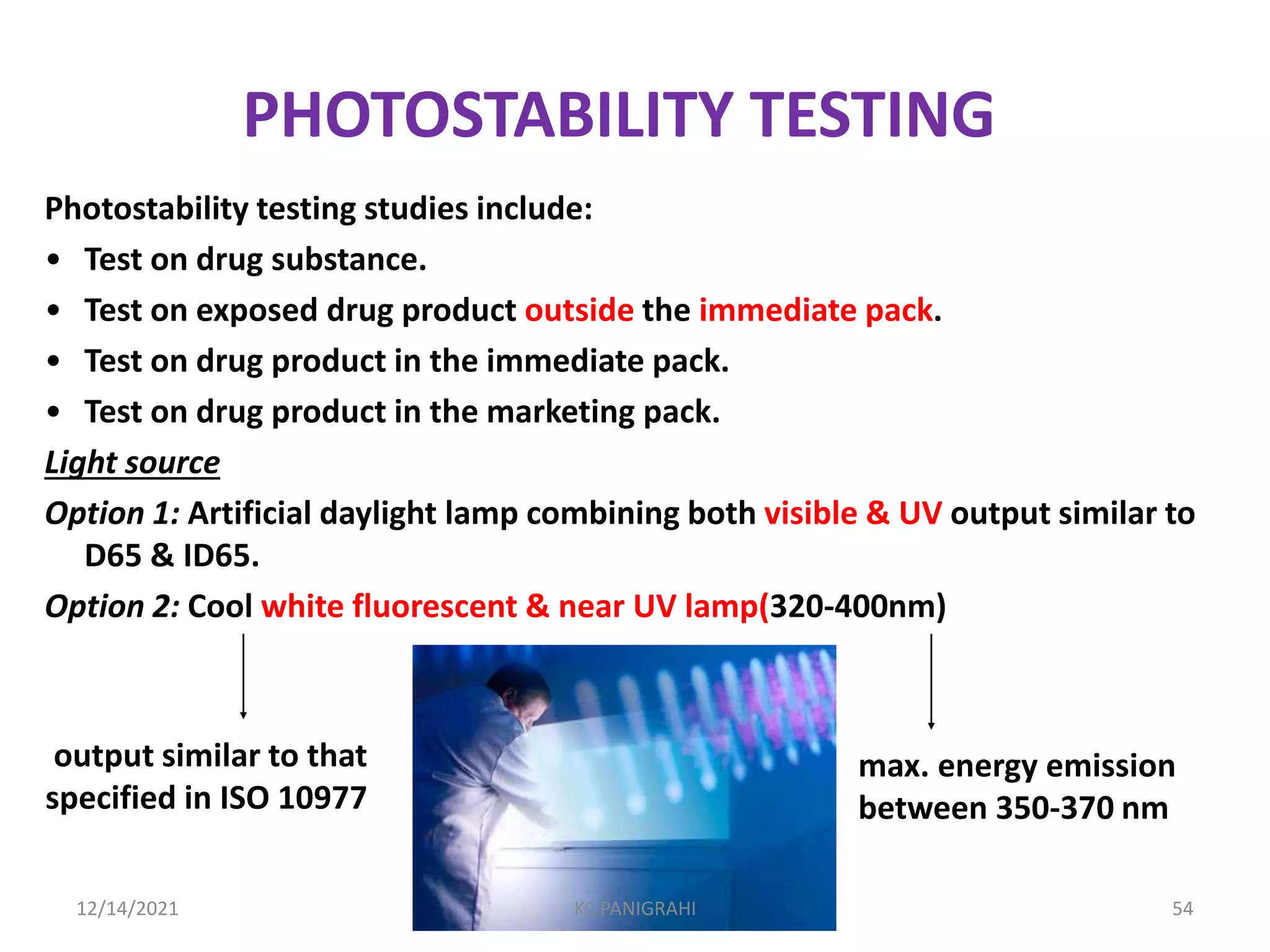
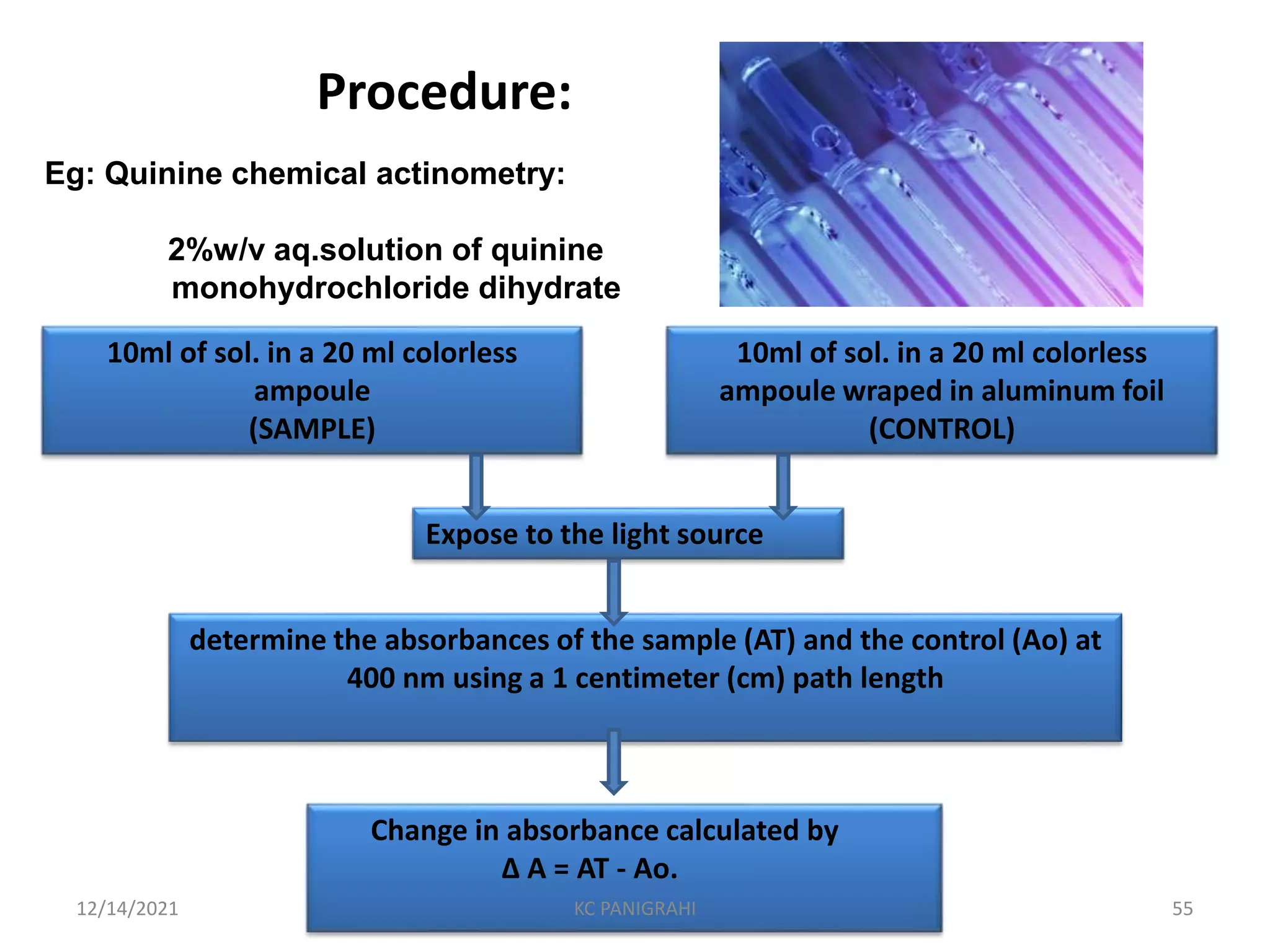
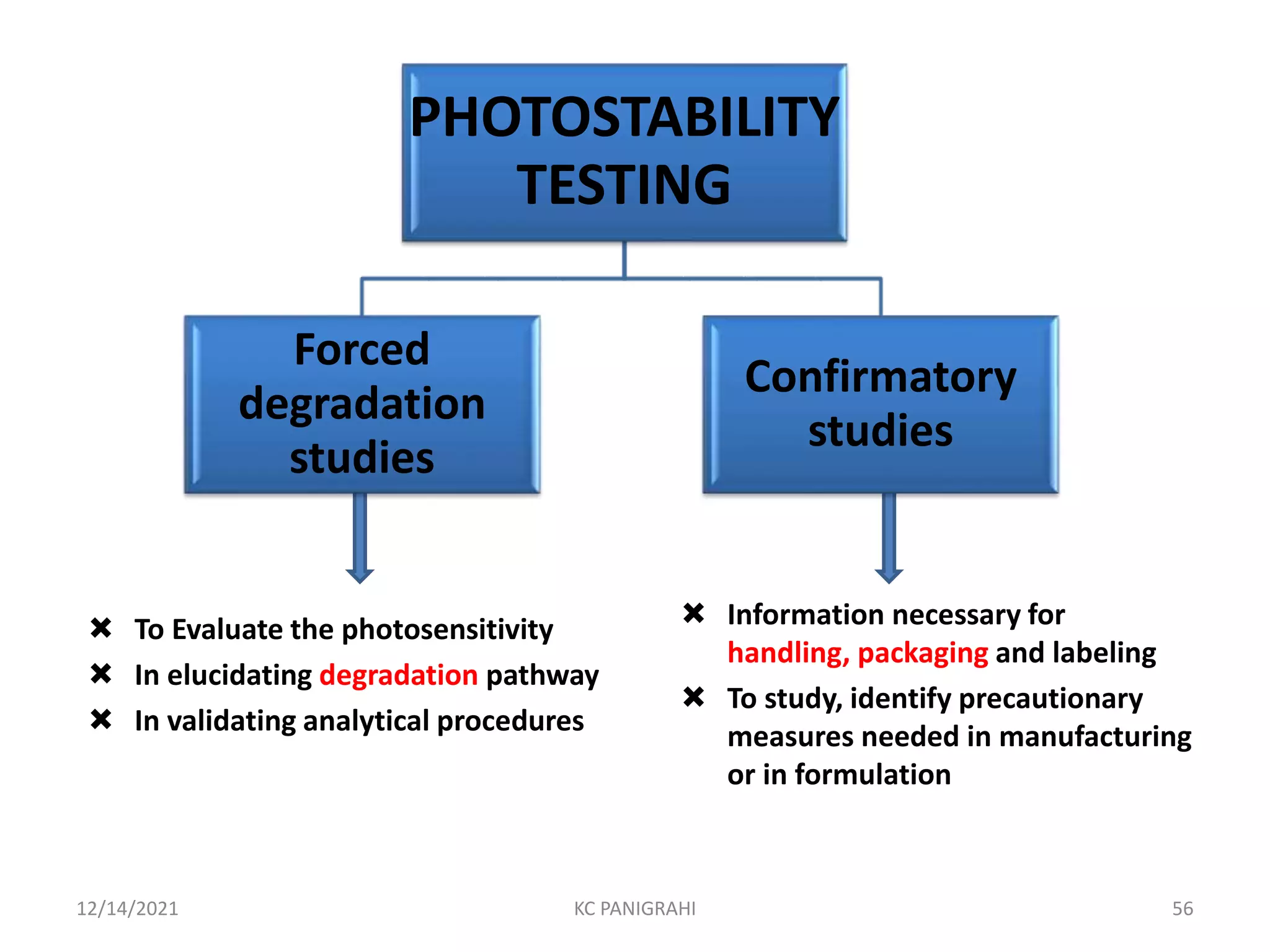
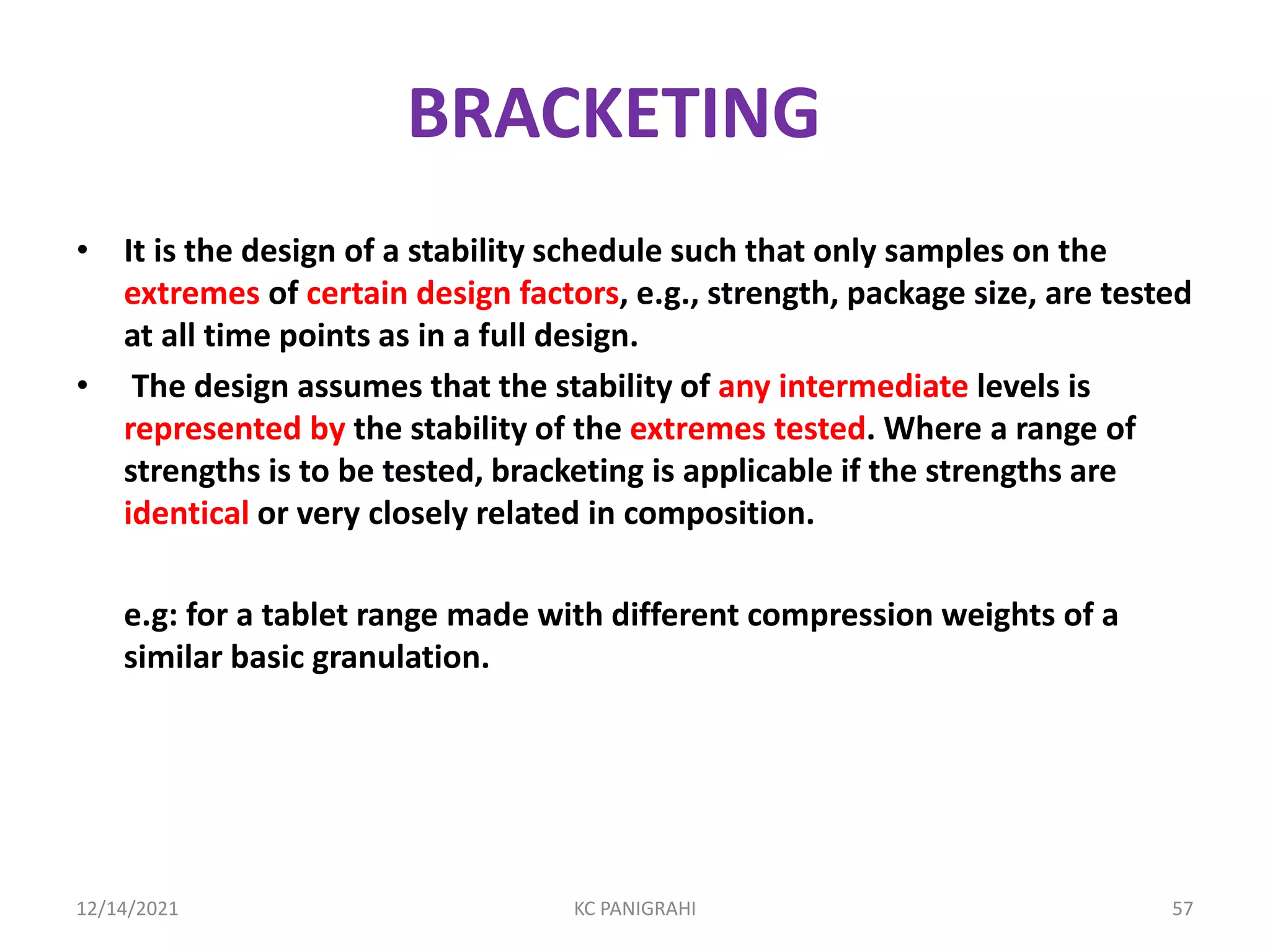
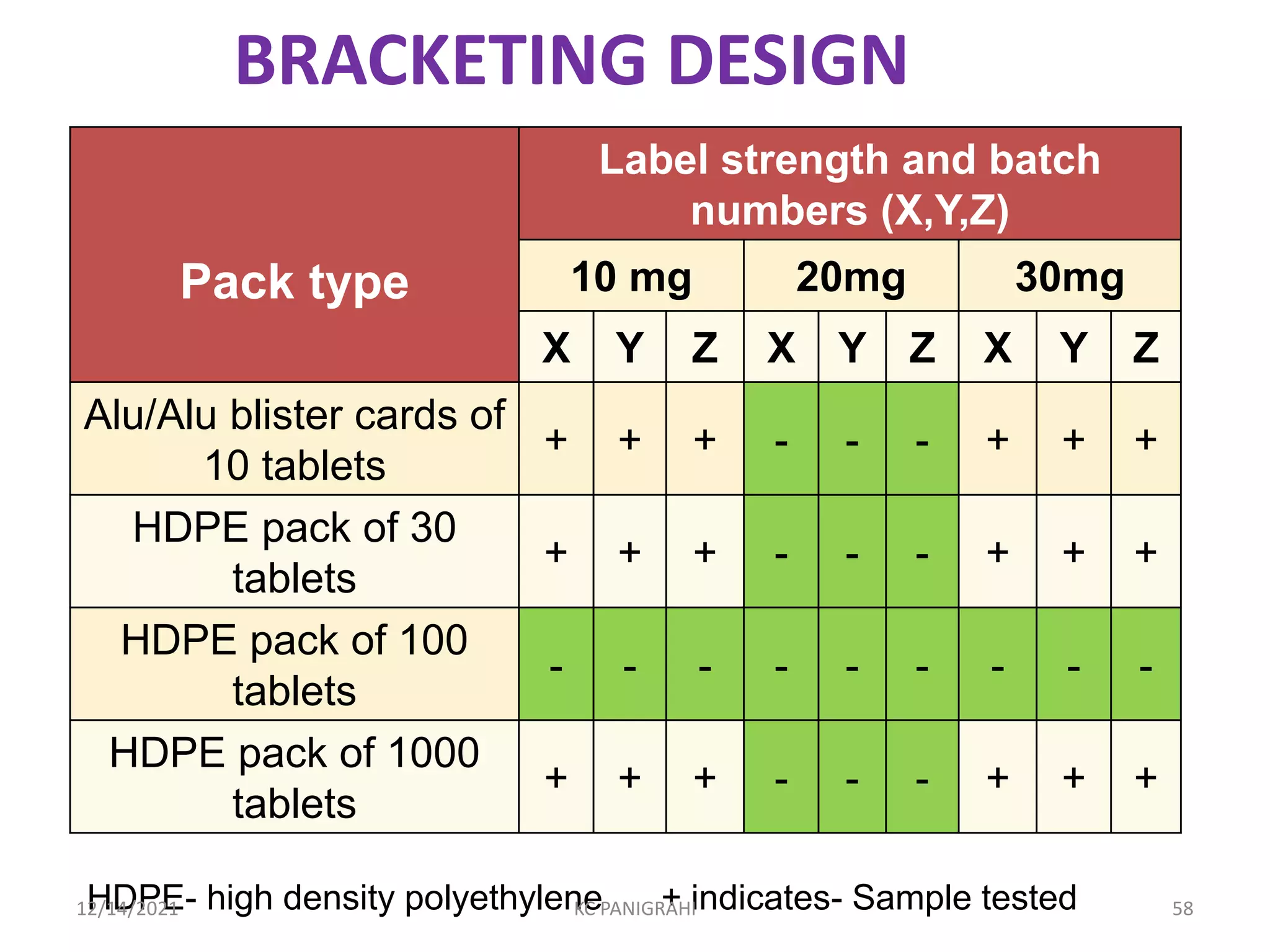
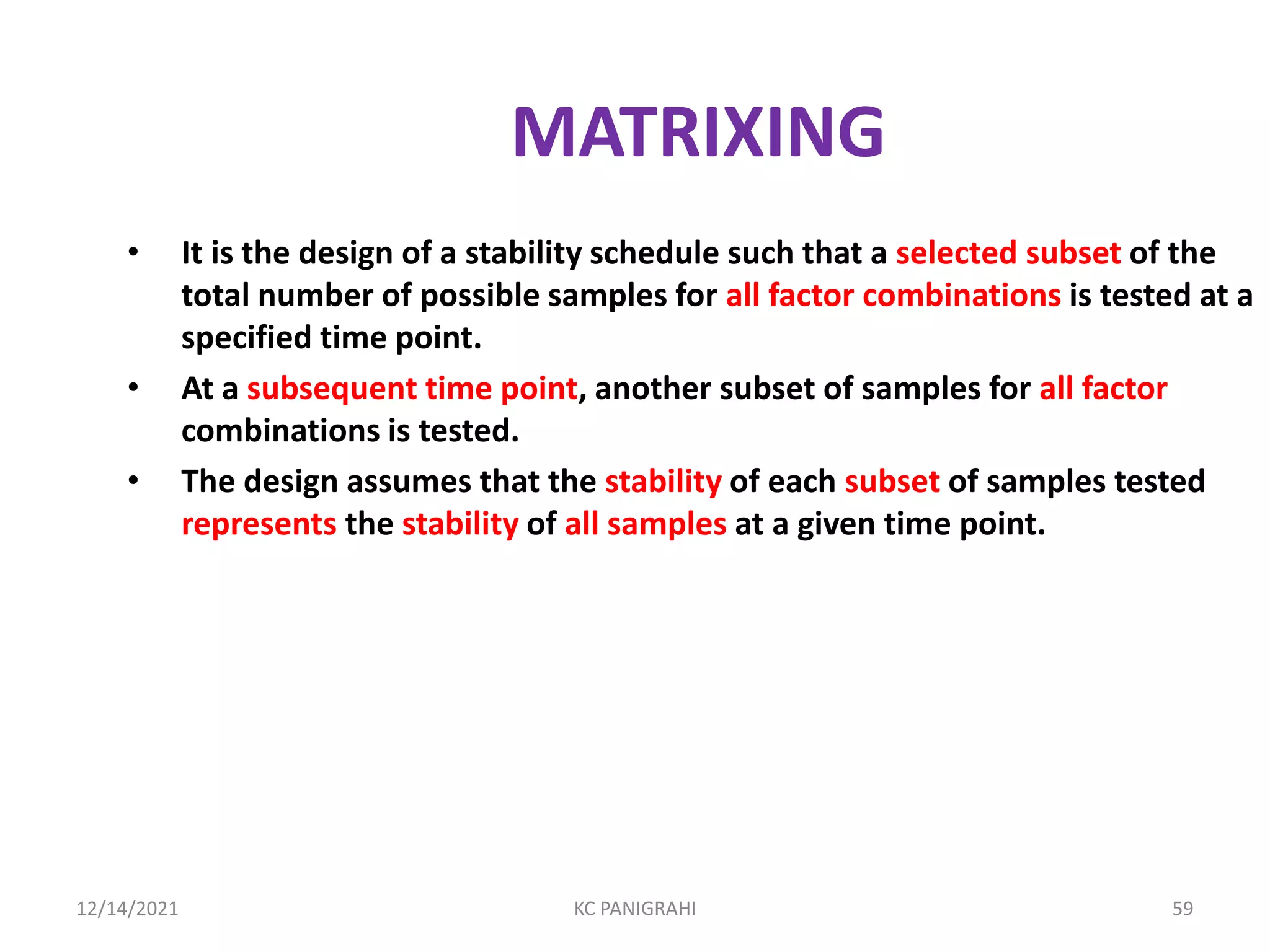
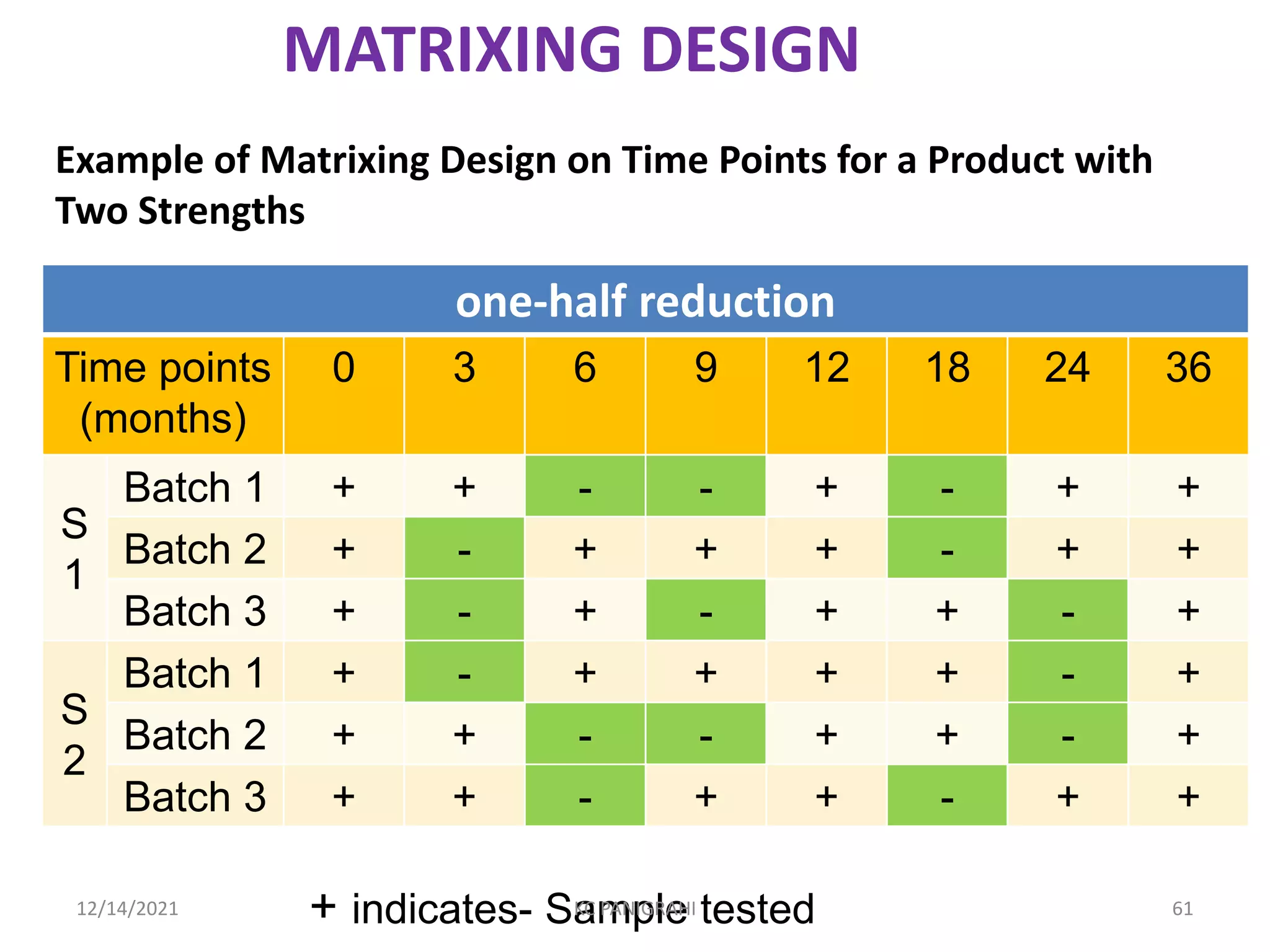
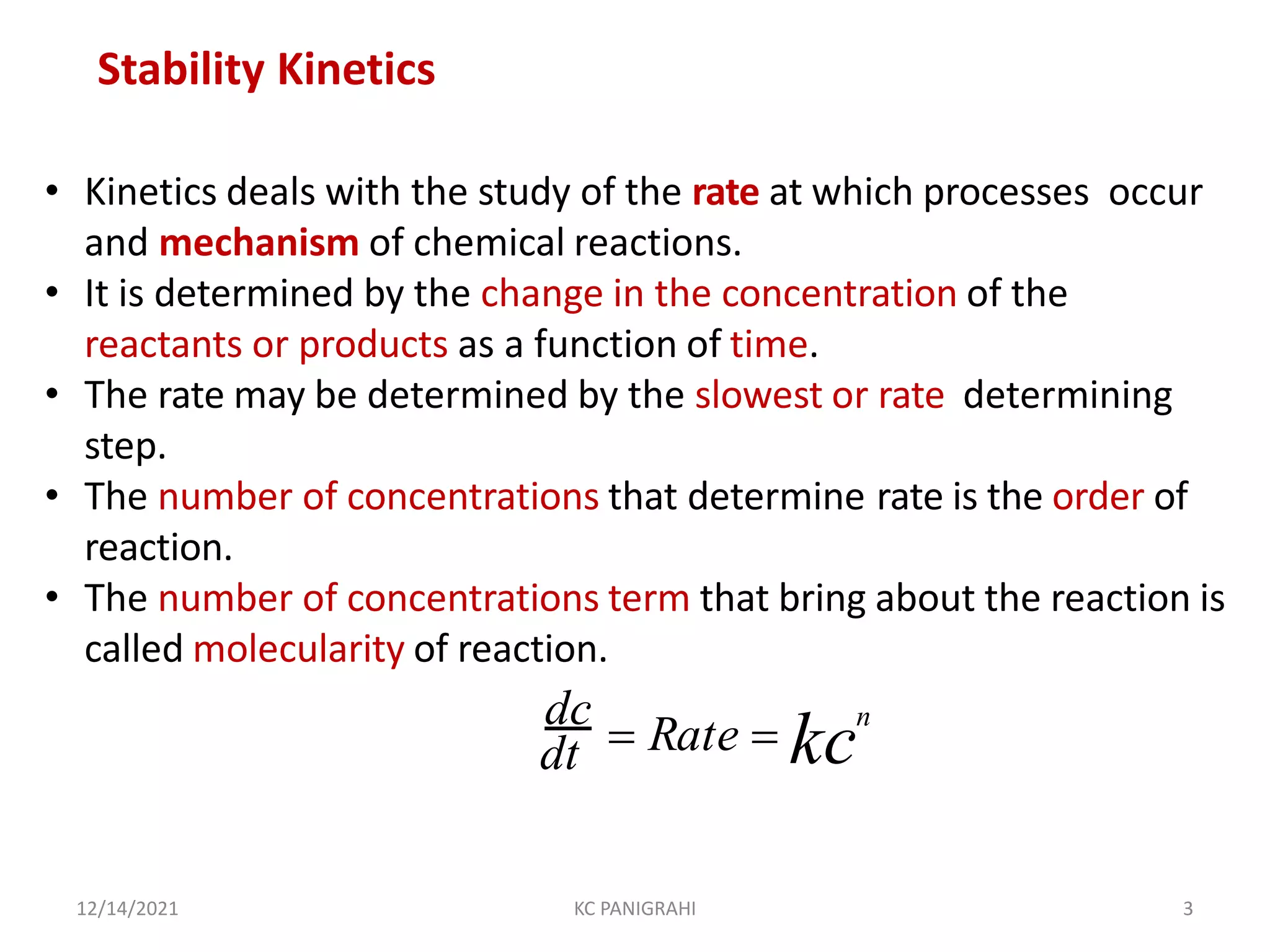
The document discusses kinetics of stability and accelerated stability testing. It provides details on zero order, first order and second order reactions. It explains the determination of rate constants, half life and time for 90% degradation using kinetic equations. The document also discusses Arrhenius equation for predicting shelf life from accelerated stability studies conducted at elevated temperatures. It summarizes the guidelines for stability testing of active pharmaceutical ingredients and finished pharmaceutical products as per ICH.


![ZERO ORDER REACTION:
Rate is constant and is independent of the concentration of any of the reactants. A
constant rate of drug release from a dosage form is highly desirable.
Reactant [A] Product(P)
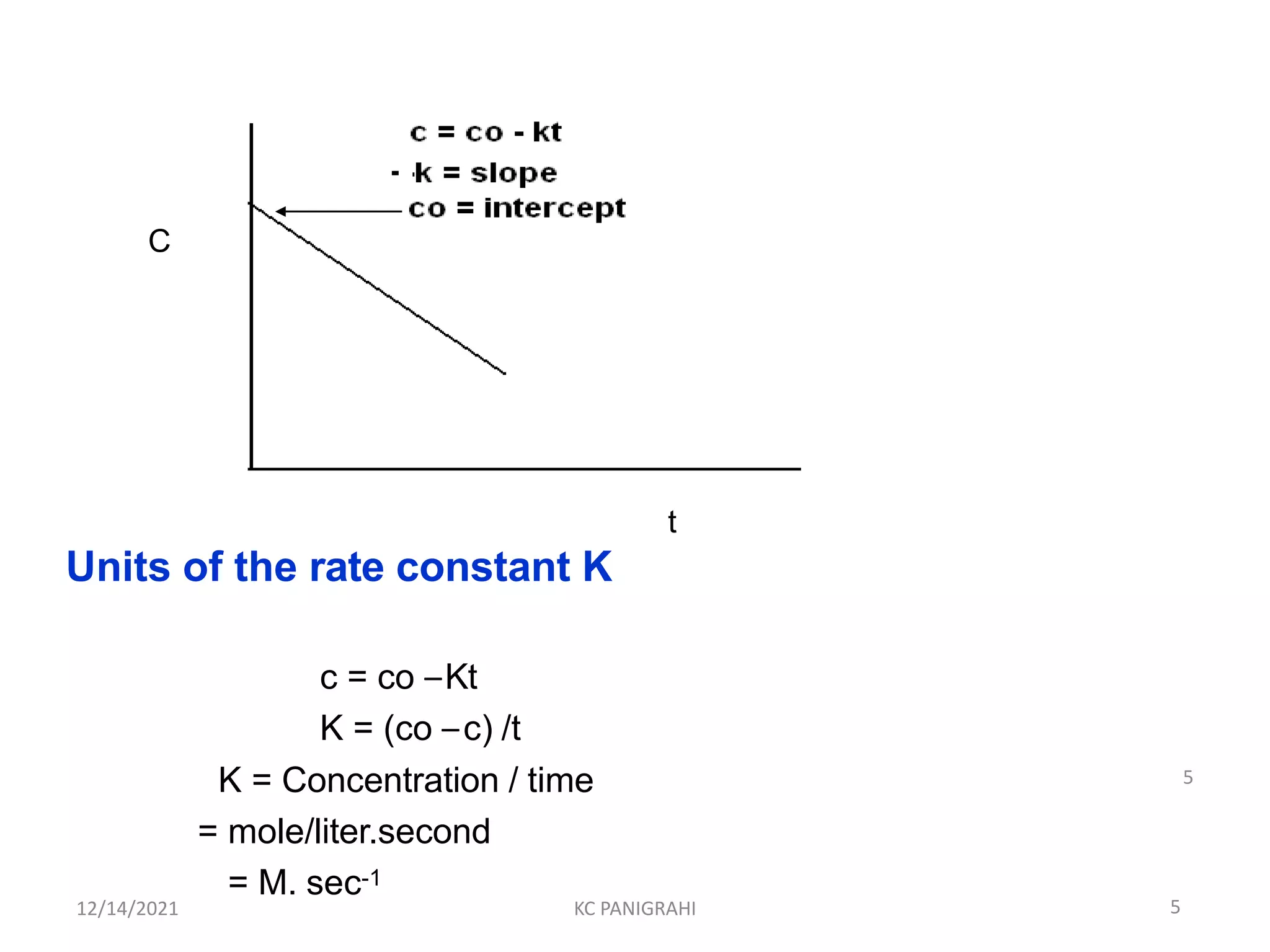
Rate = - dc/dt = K [c]0
- dc/dt = k
dc = - k dt
t
ct
dc kdt
c0 t0
co = Initial concentration
ct = Concentration at time
Ct –C0 = -kt
12/14/2021 KC PANIGRAHI 4](https://image.slidesharecdn.com/stabilitykineticsandtesting-211214060825/75/Stability-kinetics-and-testing-4-2048.jpg)