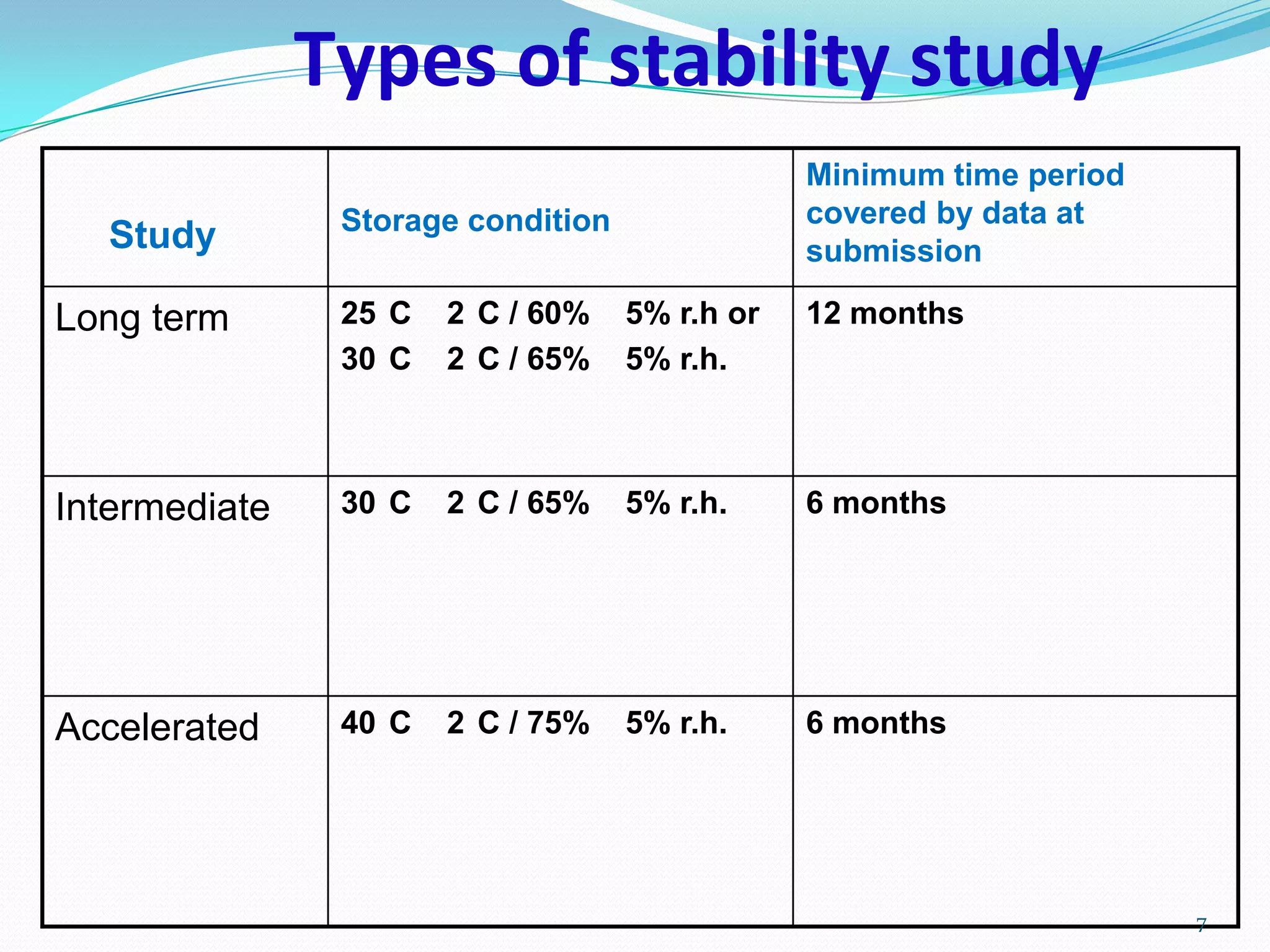
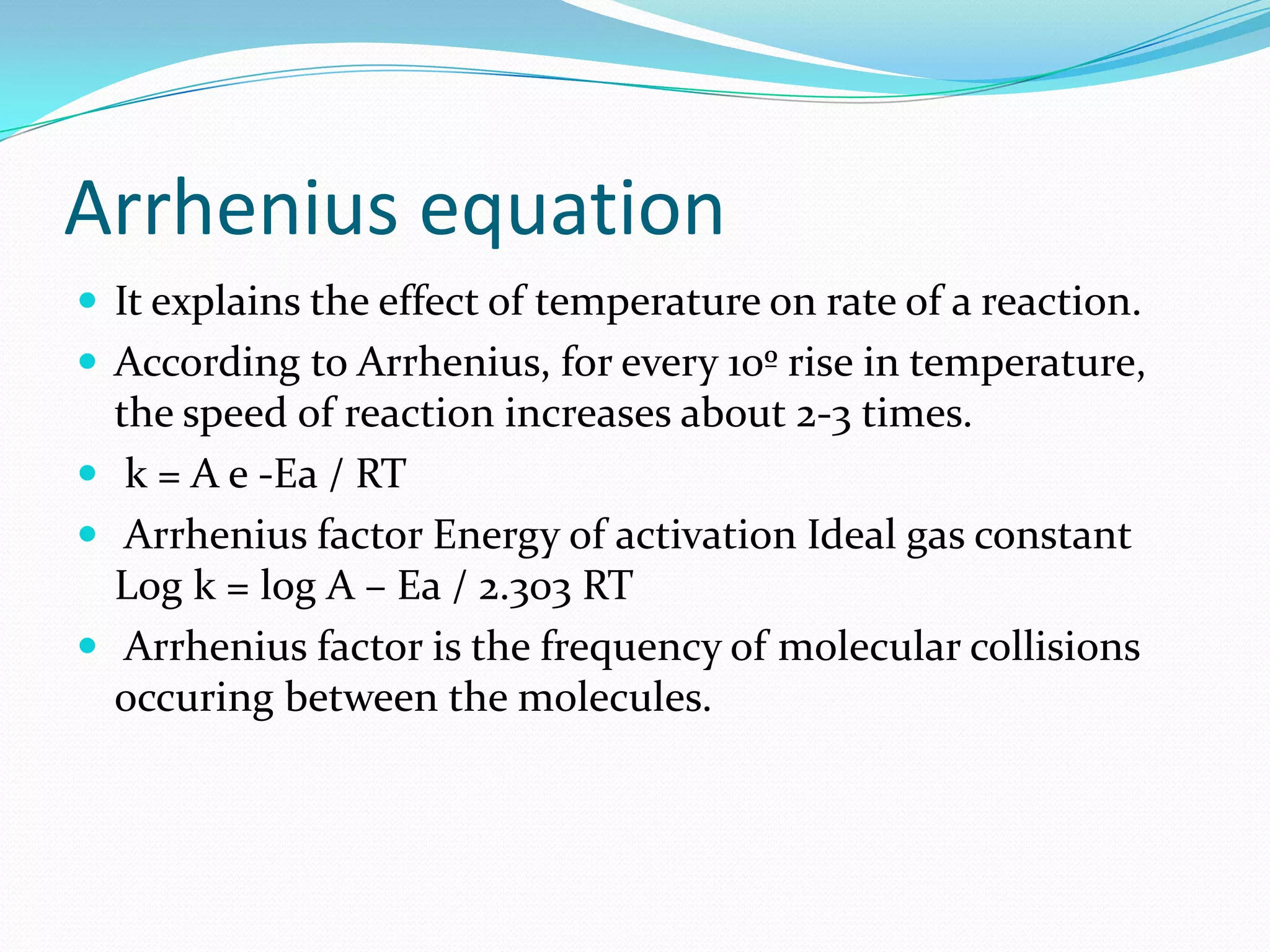
This document discusses stability testing and shelf life prediction of pharmaceutical products. It defines stability as a product remaining within specifications over its shelf life under recommended storage conditions. Stability testing determines a product's shelf life and recommended storage. Types of stability studies discussed are long term, intermediate, and accelerated. The Arrhenius equation is used to predict shelf life from accelerated studies by determining reaction kinetics at different temperatures. Packaging selection, addition of overages, and limitations of shelf life prediction are also summarized.