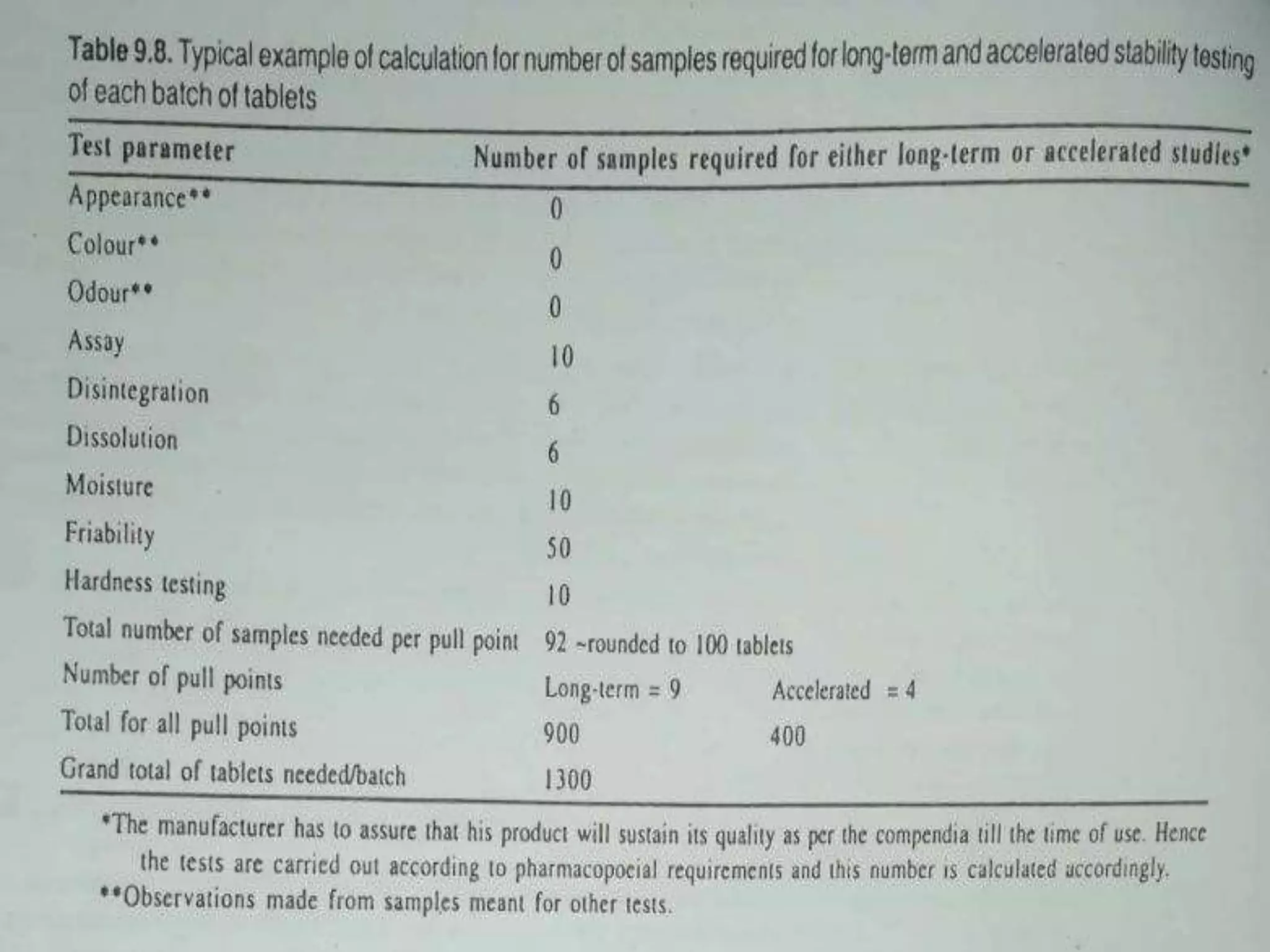
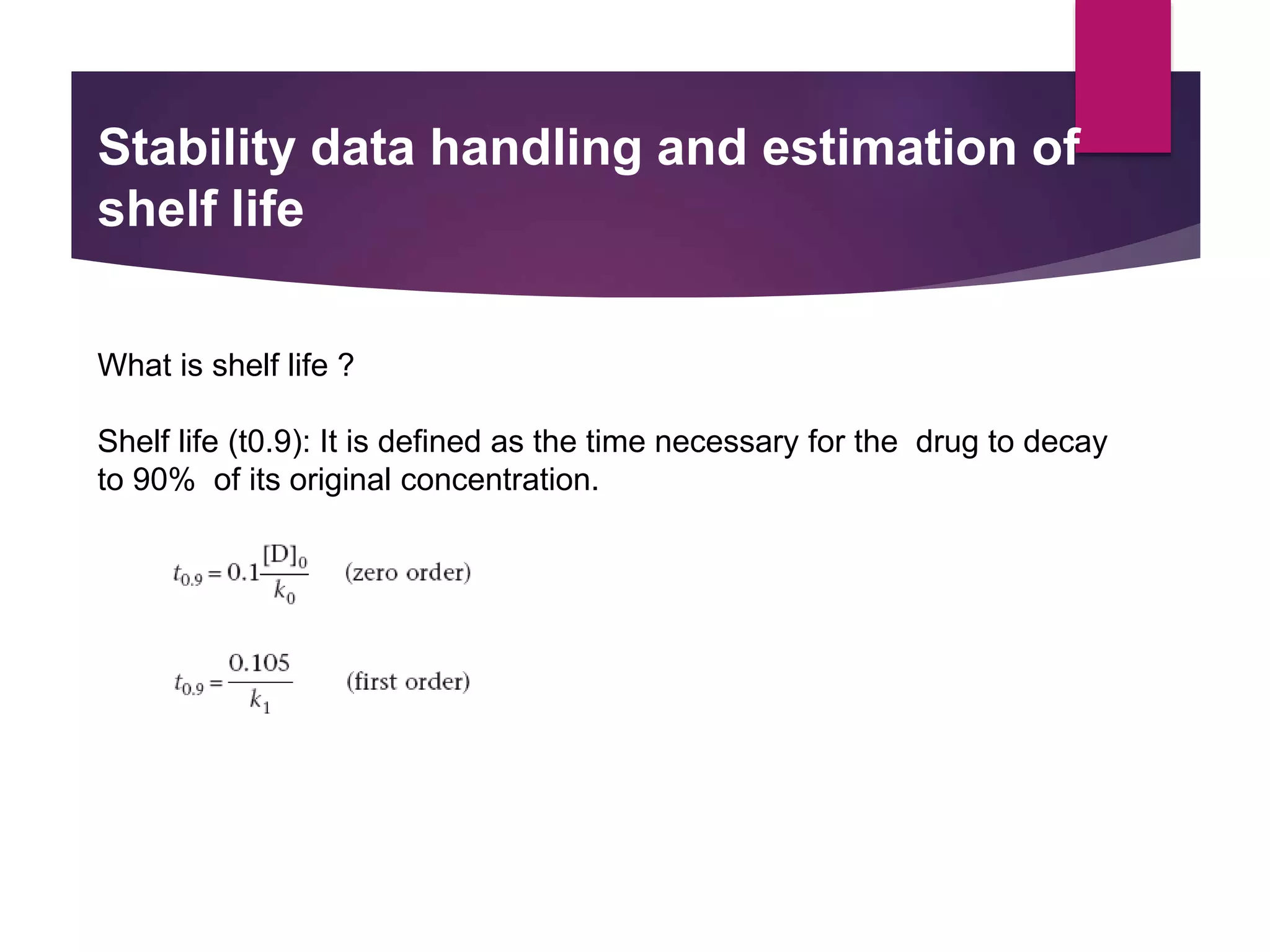
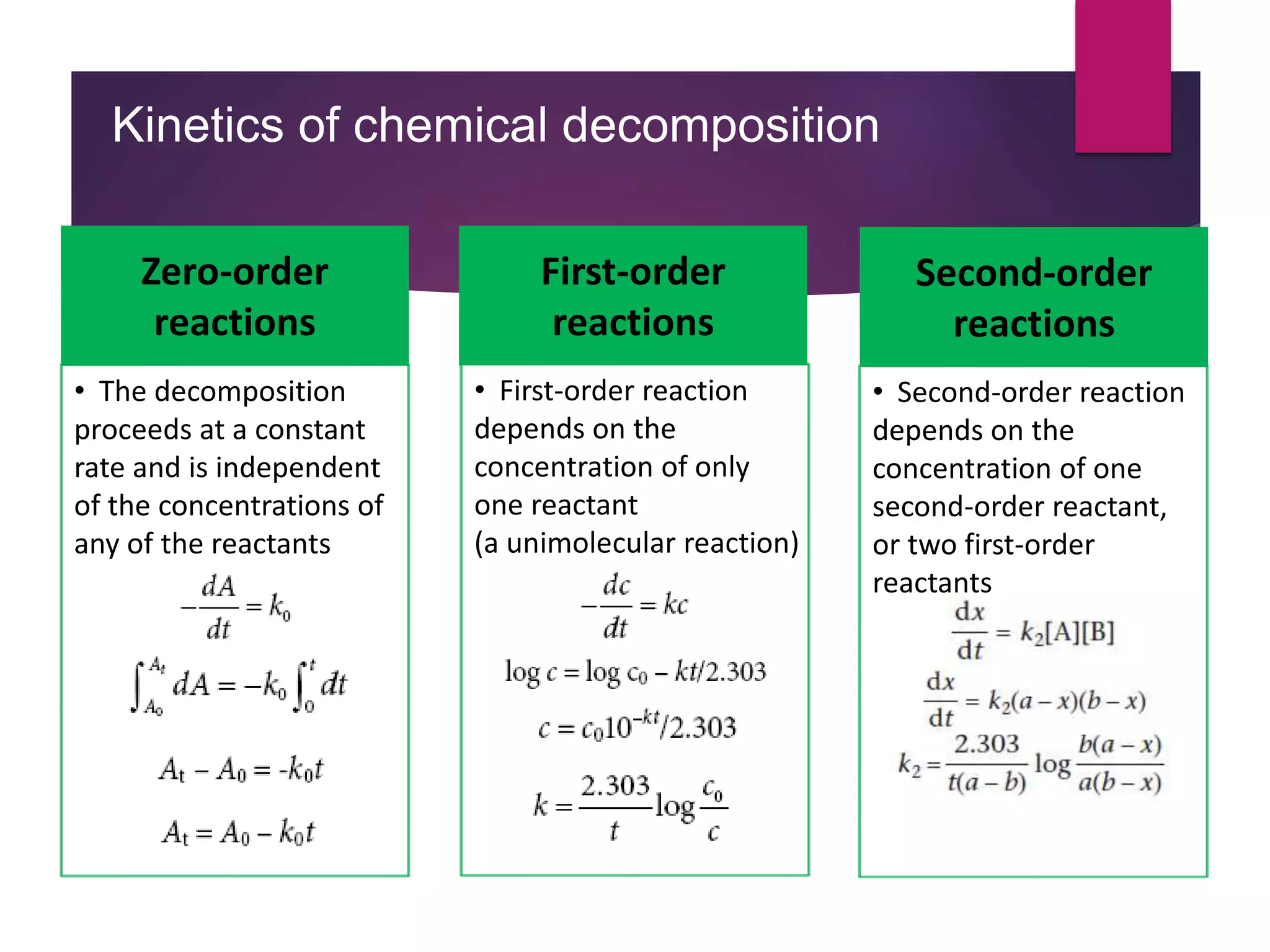
The document outlines the protocols and methods for conducting stability testing during product development, including sample preparation, data recording, and analysis for shelf life estimation. It explains shelf life definitions, kinetic reactions, testing frequencies, and how to determine shelf life through real-time and accelerated stability studies. Additionally, it emphasizes the importance of package labeling which must include expiry dates, storage instructions, and shelf life recommendations.