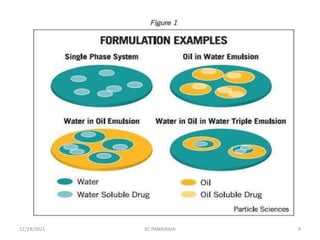
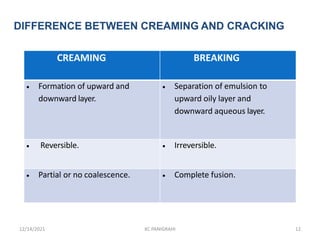
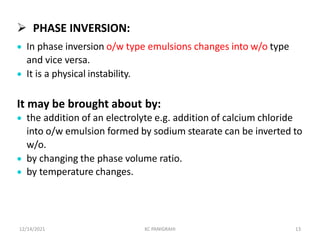
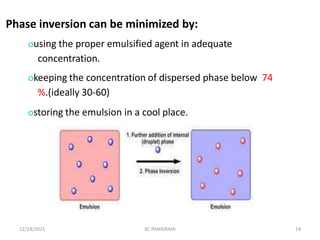
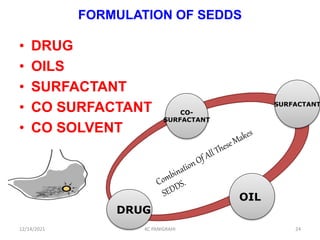
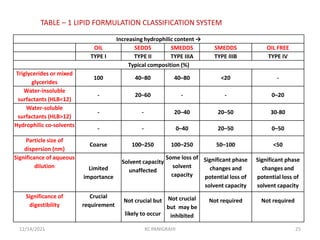
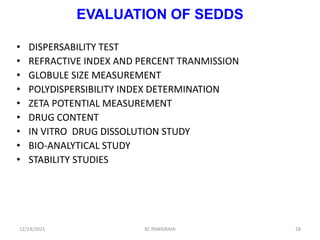
This document discusses emulsions and self-emulsifying drug delivery systems (SEDDS). It defines emulsions as mixtures of two immiscible liquids stabilized by an emulsifying agent. The main types of emulsions described are oil-in-water, water-in-oil, multiple emulsions, and microemulsions. SEDDS are defined as isotropic mixtures of oils, surfactants, and co-solvents/co-surfactants that spontaneously form emulsions when exposed to aqueous media and can improve drug solubility and bioavailability. Key factors in developing SEDDS like choice of oils, surfactants, and evaluation methods are also summarized.