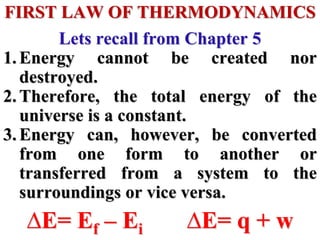
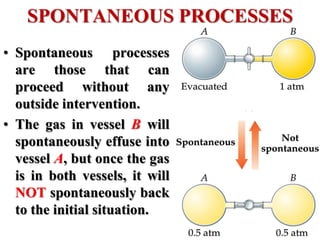
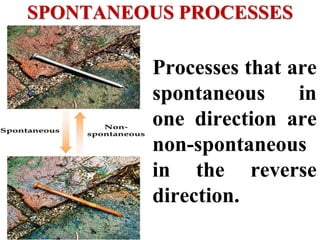
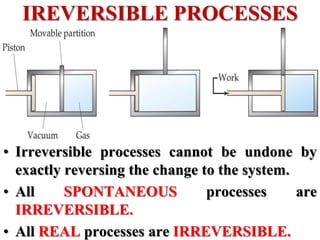
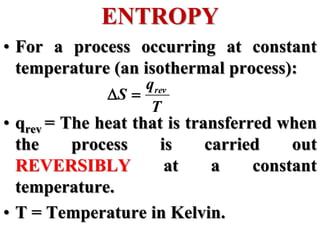
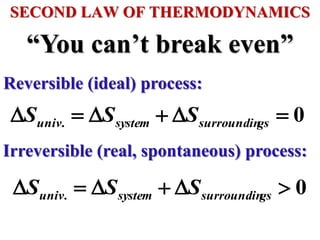
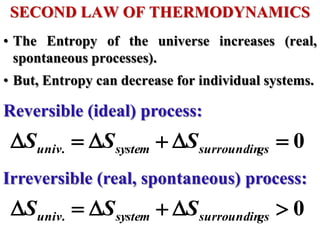
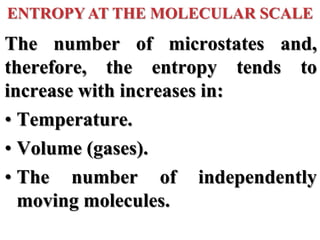
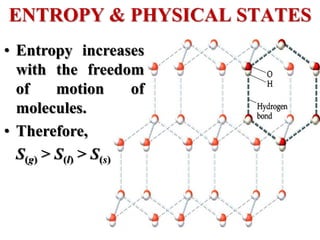
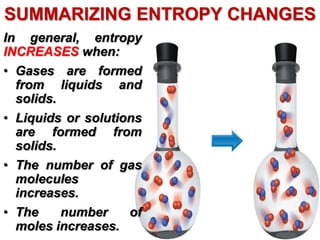
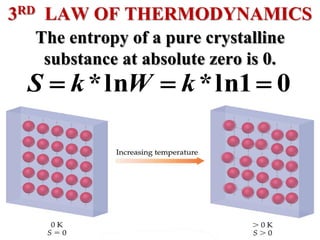
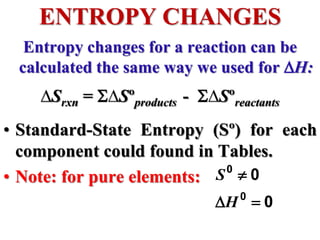
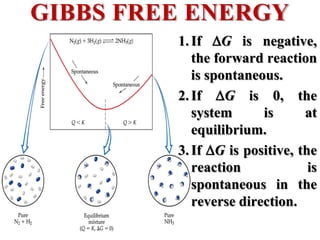
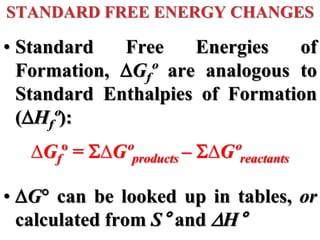
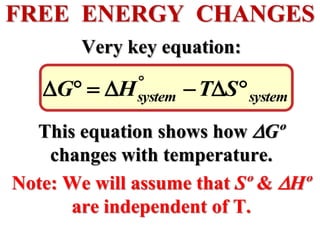
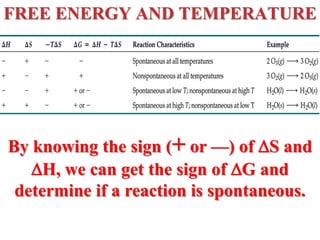
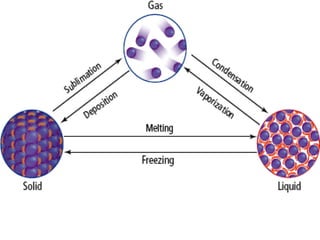
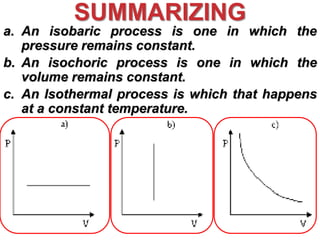
This document discusses key concepts from the first, second, and third laws of thermodynamics. It begins by defining spontaneous and reversible processes, explaining that spontaneous processes have a natural direction while reversible processes can return a system to its initial state. It then introduces entropy, describing how it increases for spontaneous processes based on the second law of thermodynamics. The document also discusses entropy at the molecular level and how it relates to temperature, phase changes, and the number of possible microscopic arrangements. Finally, it covers how entropy is used to define free energy and how free energy changes can indicate whether a process is spontaneous.