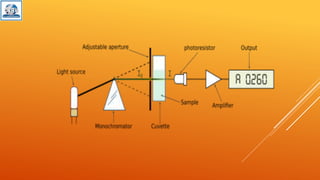
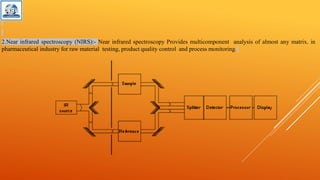
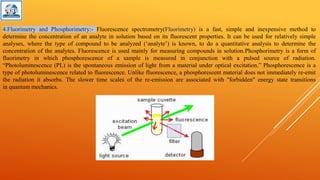
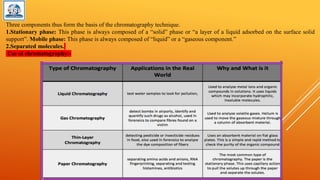
The document discusses various analytical techniques used to determine the properties of chemical substances, including chemical methods, electroanalytical methods, spectroscopy, and chromatography. Each technique, such as titrimetric methods, potentiometry, voltammetry, and chromatographic methods, has specific applications and instruments utilized for analysis. The content is tailored for a deeper understanding of analytical chemistry methods in a laboratory setting.