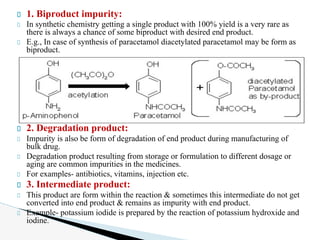
The document discusses the various types of impurities in pharmaceutical substances, highlighting that absolute purity is unattainable. It identifies several sources of impurities, including byproduct and degradation products, manufacturing methods, and improper storage conditions. Additionally, it emphasizes the importance of controlling impurities to ensure the safety and efficacy of pharmaceuticals.