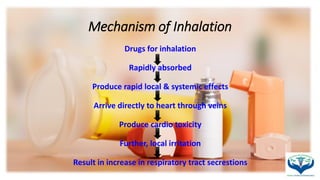
1. The document discusses various gases used as inhalants including oxygen, carbon dioxide, and nitrous oxide. It describes their chemical and physical properties, methods of preparation, storage requirements, assays for testing purity, and pharmaceutical uses.
2. Key pharmaceutical uses include oxygen as an inhalant to support respiration, carbon dioxide as a respiratory stimulant and to promote absorption, and nitrous oxide as a general anesthetic along with oxygen, especially in dental procedures.
3. Proper storage of these gases requires metal cylinders painted in identifying colors and labeled with the gas name or formula for safety.