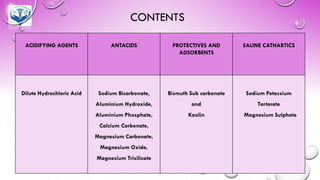
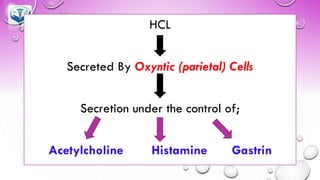
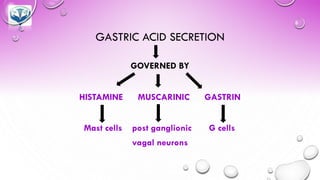
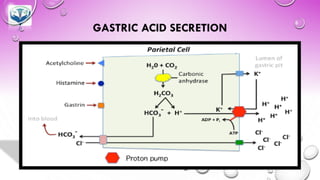
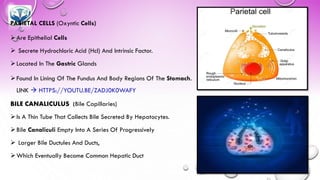
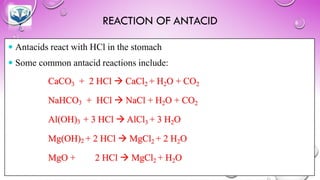
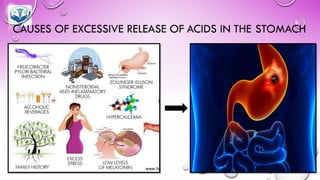
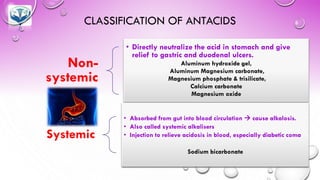
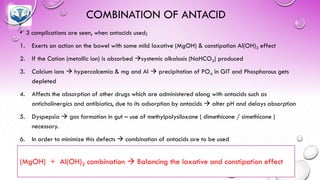
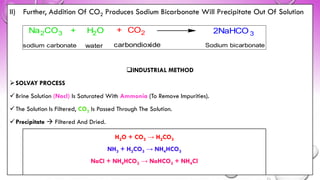
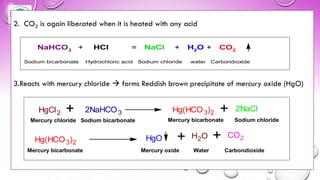
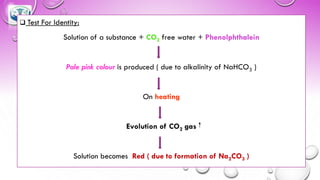
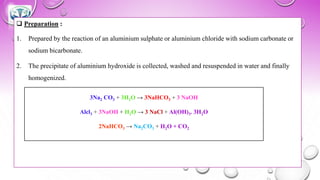
The document discusses various gastrointestinal agents used to treat disorders of the gastrointestinal tract. It describes acidifying agents and antacids which are used to alter gastric pH. Antacids work by neutralizing excess stomach acid through chemical reactions. Common antacids discussed include aluminum hydroxide, calcium carbonate, and sodium bicarbonate. The document also examines how antacids are prepared, their properties, uses, and side effects.









































![2. When heated to redness → give MgO
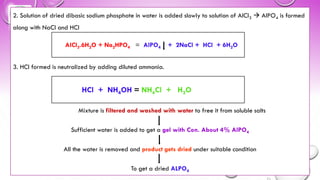
3[MgCO3.Mg(OH)2.5H2O] 4MgO + 3CO2 + 6H2O
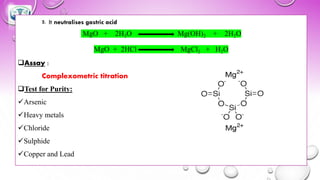
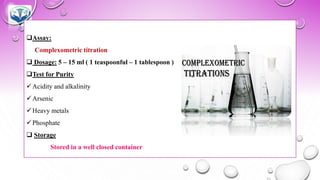
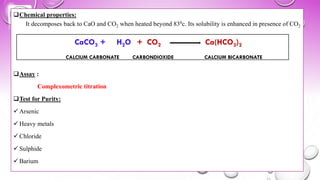
❑Assay :
Complexometric titration
❑Test for Purity:
✓Arsenic
✓Heavy metals
✓Chloride
✓Sulphide
✓Copper and Lead](https://image.slidesharecdn.com/gastrointestinalagents-210422035115/85/Gastrointestinal-agents-60-320.jpg)