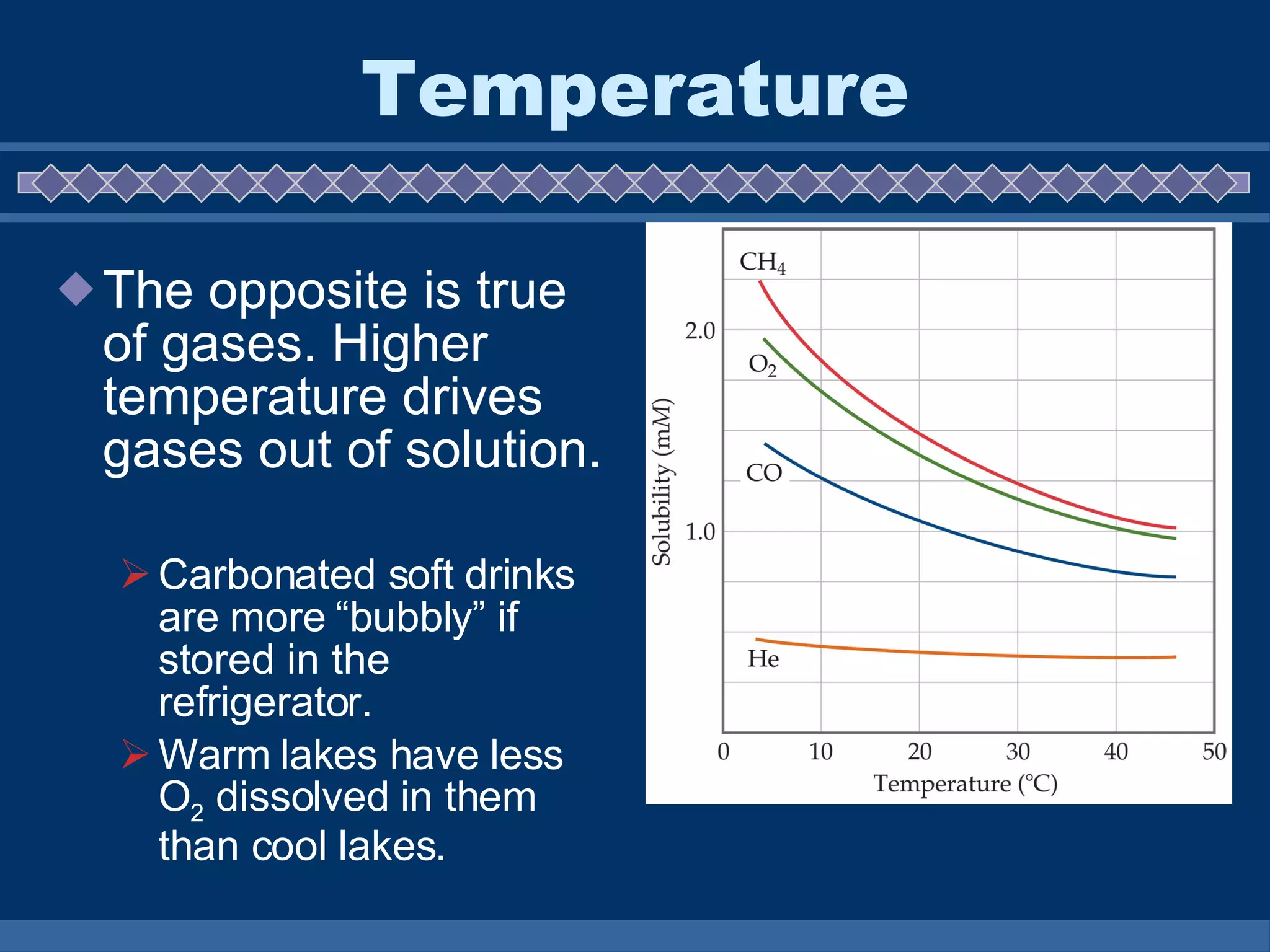
A solution is a mixture of two or more substances that are evenly distributed throughout. Solutions form when solvent molecules surround solute ions or molecules, with water being the most common solvent. The rate of solubility is affected by factors like temperature, surface area, movement, and pressure, with temperature generally increasing solid solubility but decreasing gas solubility in liquids.