Embed presentation
Download to read offline


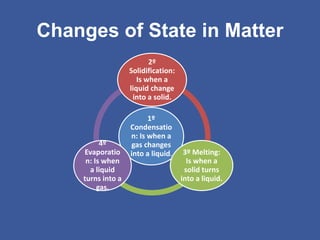
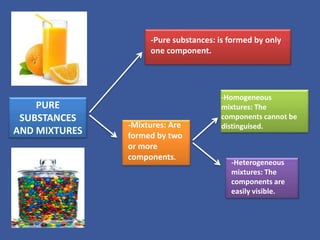
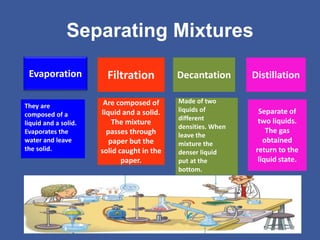

This document discusses different states of matter and changes between states. It describes solids, liquids, and gases, and the processes of condensation, solidification, melting, and evaporation. It also defines pure substances as made of one component, while mixtures contain two or more components. Homogeneous mixtures have components that cannot be distinguished, while heterogeneous mixtures have visible components. Finally, it lists some common separation methods for mixtures like evaporation, filtration, decantation, and distillation.




