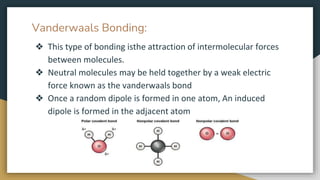
This document discusses the different types of bonding in solids. There are two main categories - primary bonds and secondary bonds. Primary bonds include ionic bonding, where positive and negative ions attract; covalent bonding, where atoms share electrons; and metallic bonding, where loosely held electrons bond atoms. Secondary bonds are weaker and include hydrogen bonding between polar molecules and van der Waals bonding from induced dipoles between neutral molecules. Examples are provided for each type of bonding.