Embed presentation

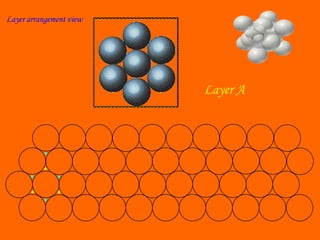

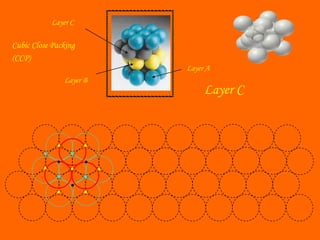



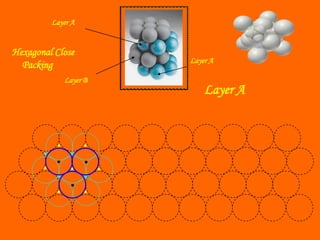


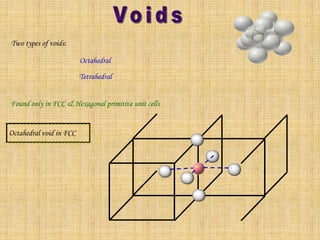
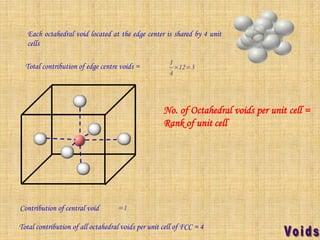

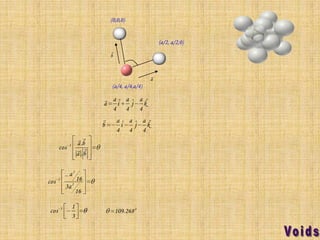
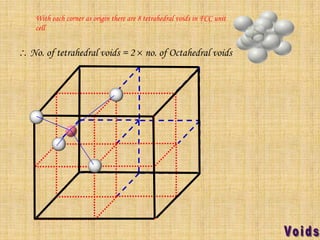
This document summarizes different types of crystal systems and lattice structures, including cubic, orthorhombic, rhombohedral, tetragonal, triclinic, and hexagonal systems. It also describes common Bravais lattices like primitive, FCC, and BCC. Finally, it discusses packing of layers in cubic close packing and hexagonal close packing, and defines octahedral and tetrahedral voids that can exist in FCC and hexagonal unit cells.














