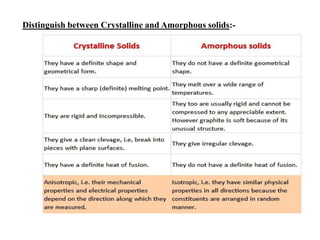
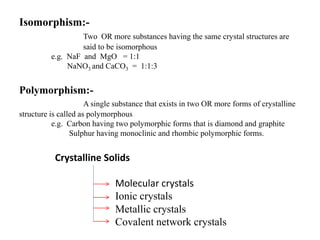
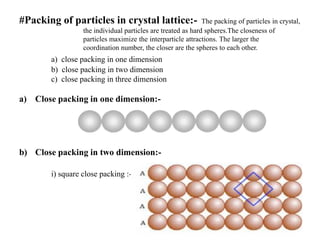
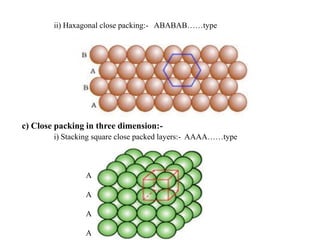
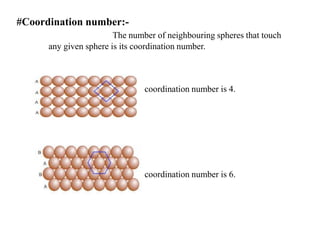
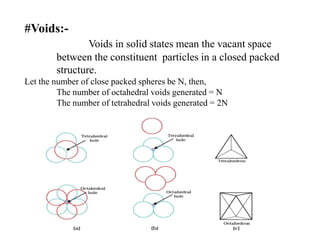
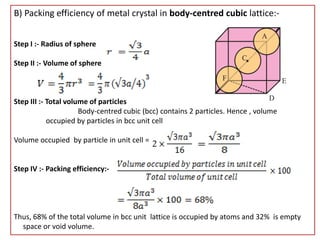
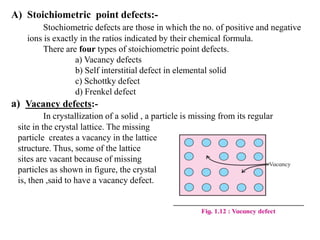
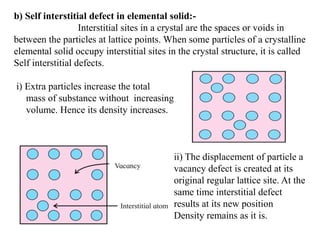
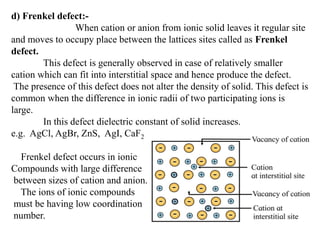
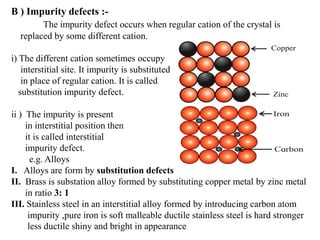
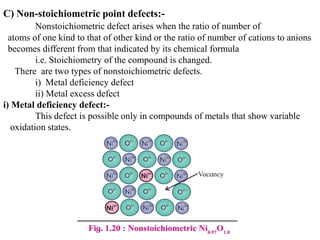
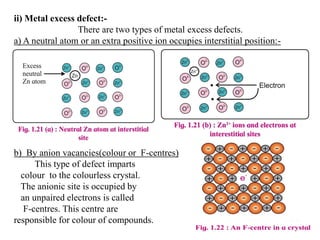
This document provides an overview of solid state chemistry. It discusses the different types of solids including crystalline and amorphous solids. Crystalline solids are further classified based on their crystal structures. The key crystal structures discussed are the cubic system including simple cubic, body-centered cubic, and face-centered cubic unit cells. Methods of packing particles in crystal lattices like close packing in one, two, and three dimensions are also summarized. The document concludes with discussing common crystal defects or imperfections.