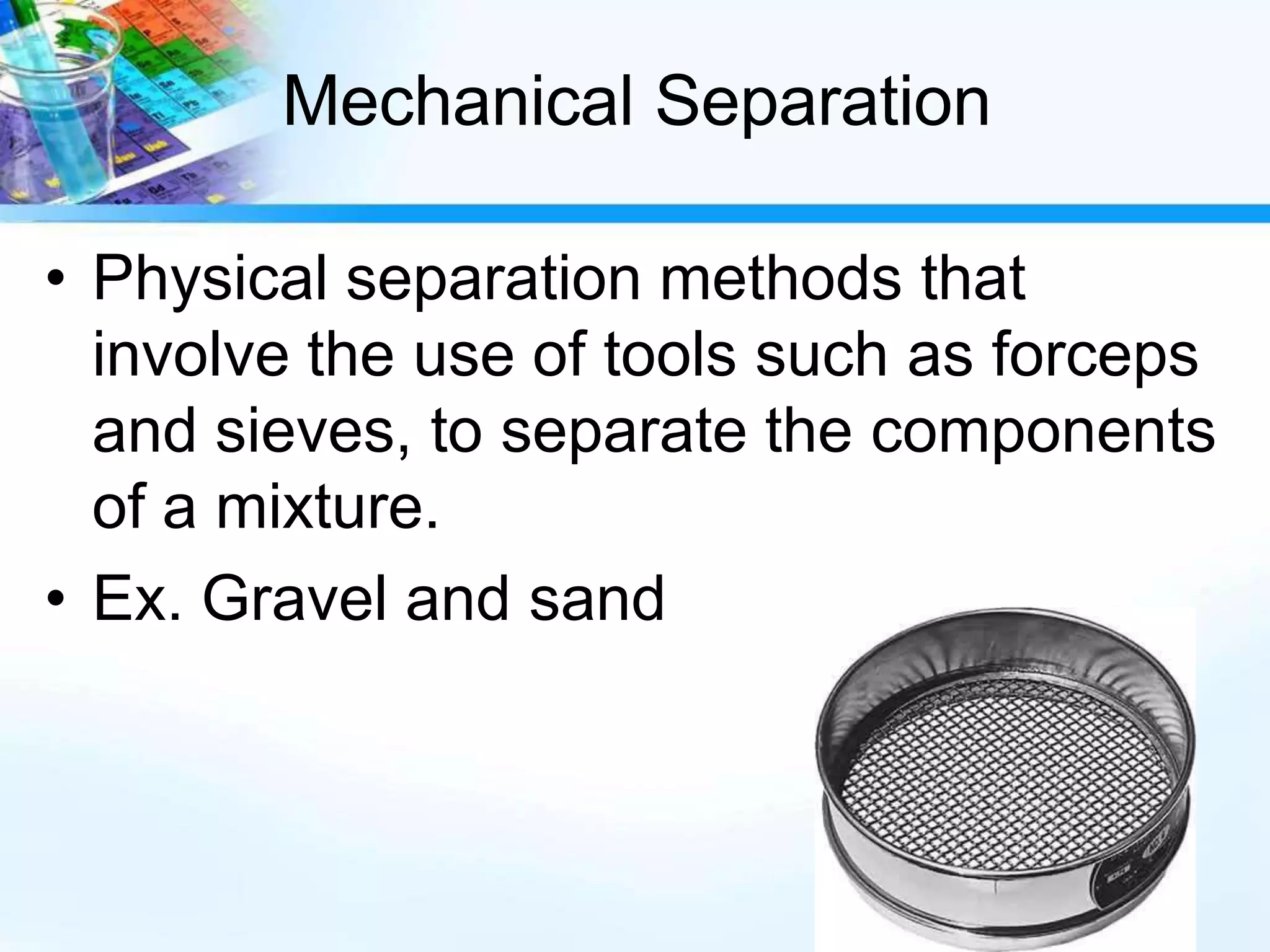
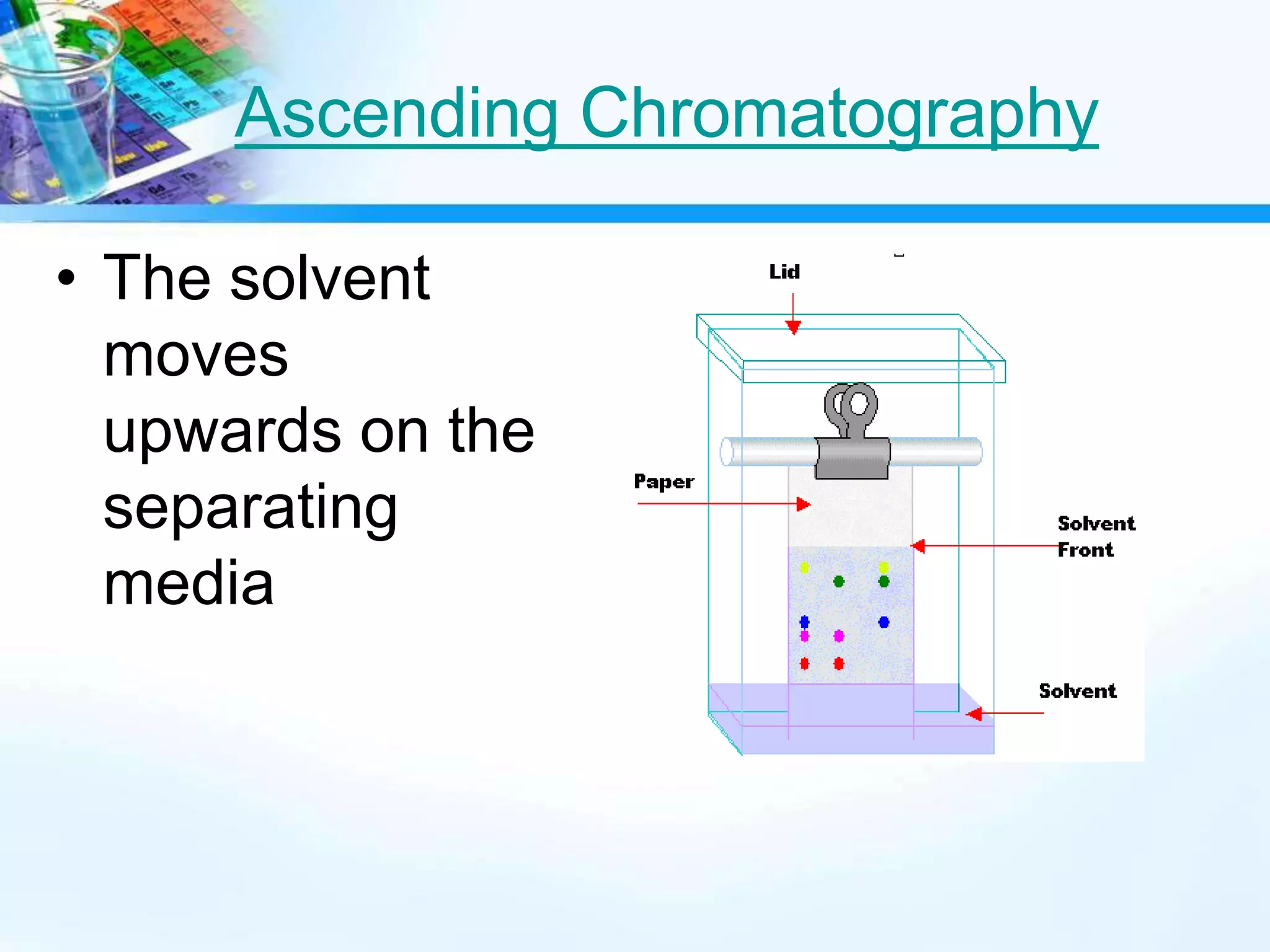
This document discusses various separation techniques used to separate mixtures into their pure components. It describes techniques such as filtration, mechanical separation, flotation, centrifugation, distillation, crystallization, and chromatography. Distillation techniques include simple distillation and fractional distillation, which separate mixtures based on differences in boiling points. Chromatography separates soluble substances using a mobile phase and stationary phase. Examples are provided to illustrate how each technique separates specific mixtures.