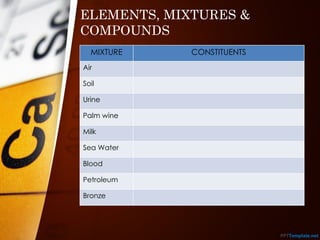
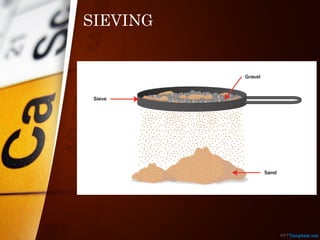
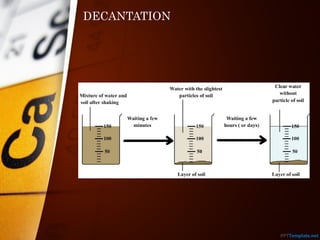
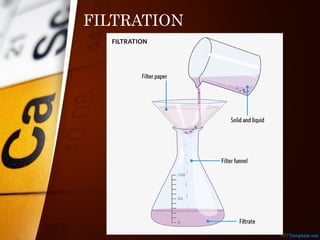
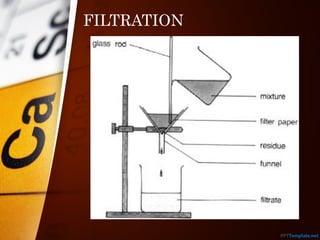
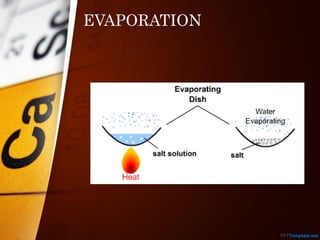
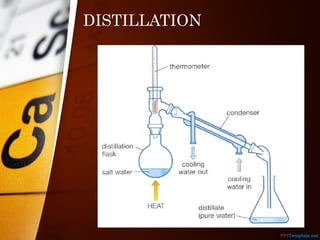
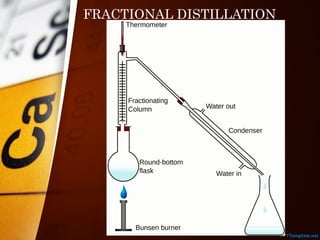
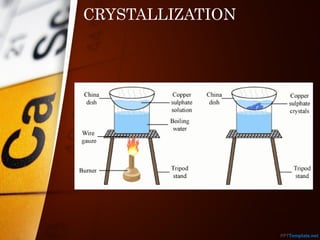
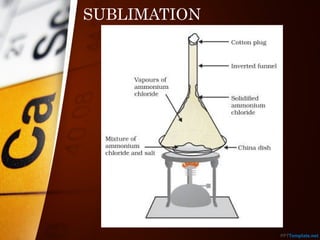
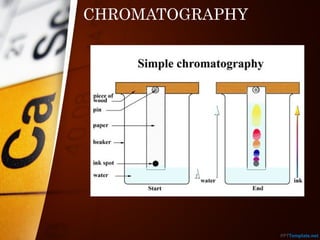
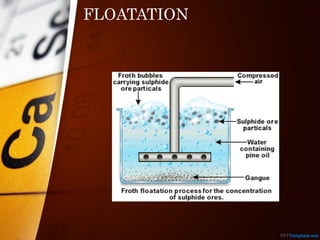
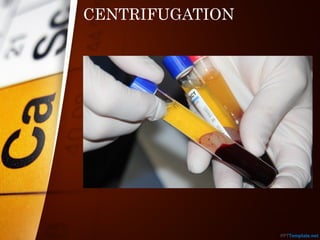
The document outlines various standard separation techniques for mixtures in chemistry, including methods such as filtration, evaporation, distillation, and chromatography, each with specific applications based on the properties of the constituents involved. It explains the differences between pure and impure substances, as well as the criteria for purity, highlighting that pure substances have consistent melting and boiling points. Additionally, it emphasizes the importance of these techniques in industrial applications and scientific analysis.