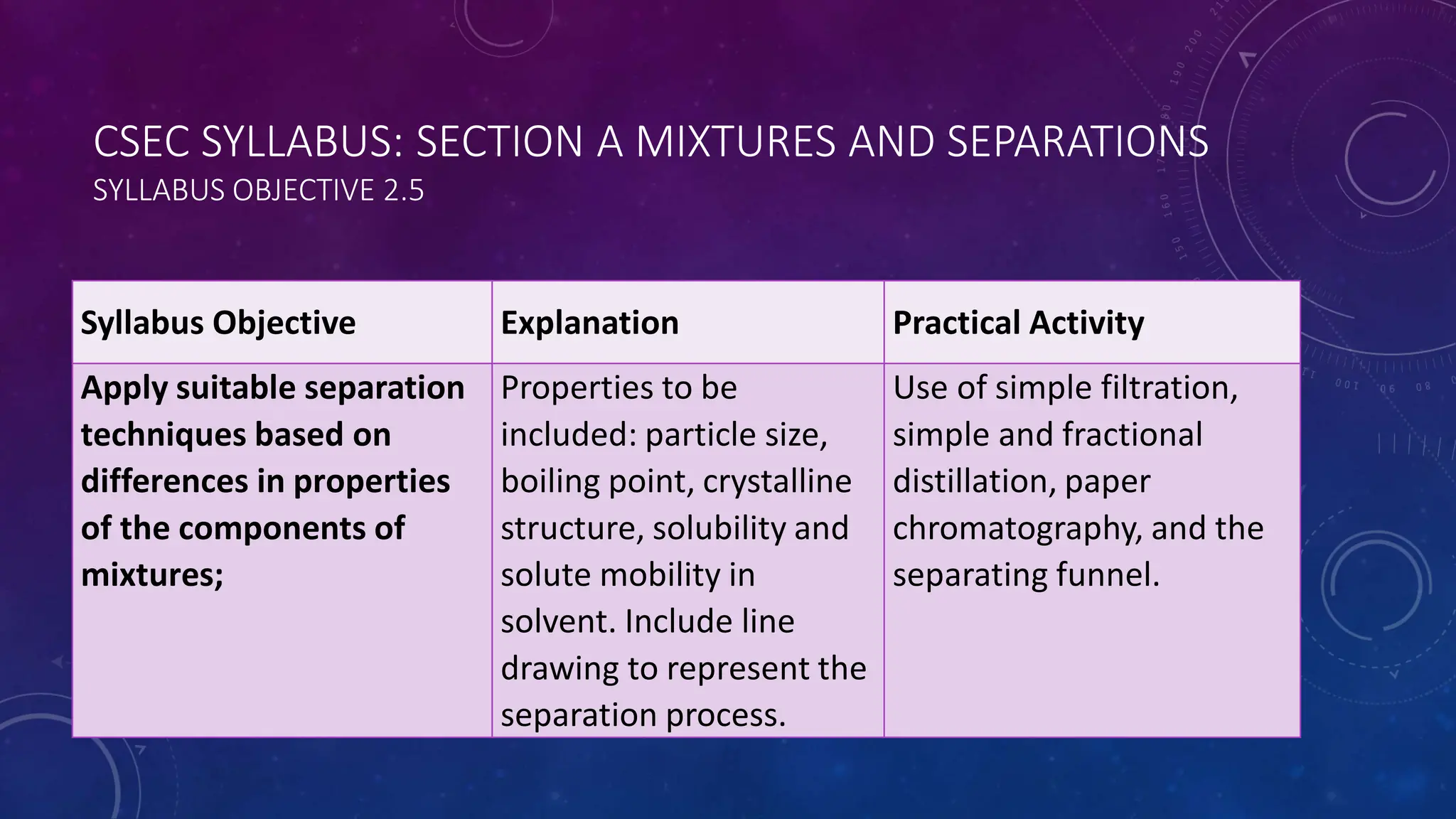
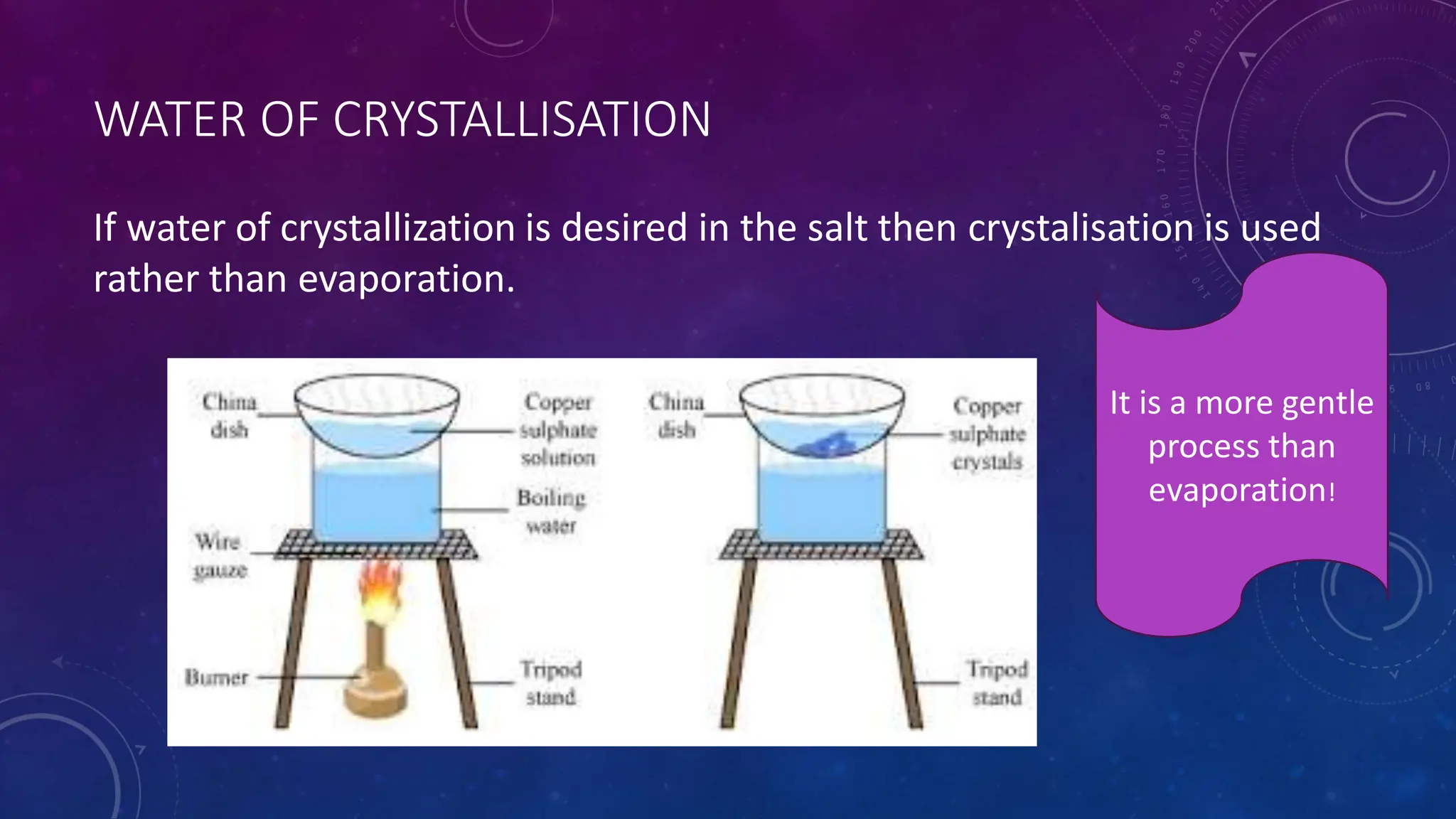
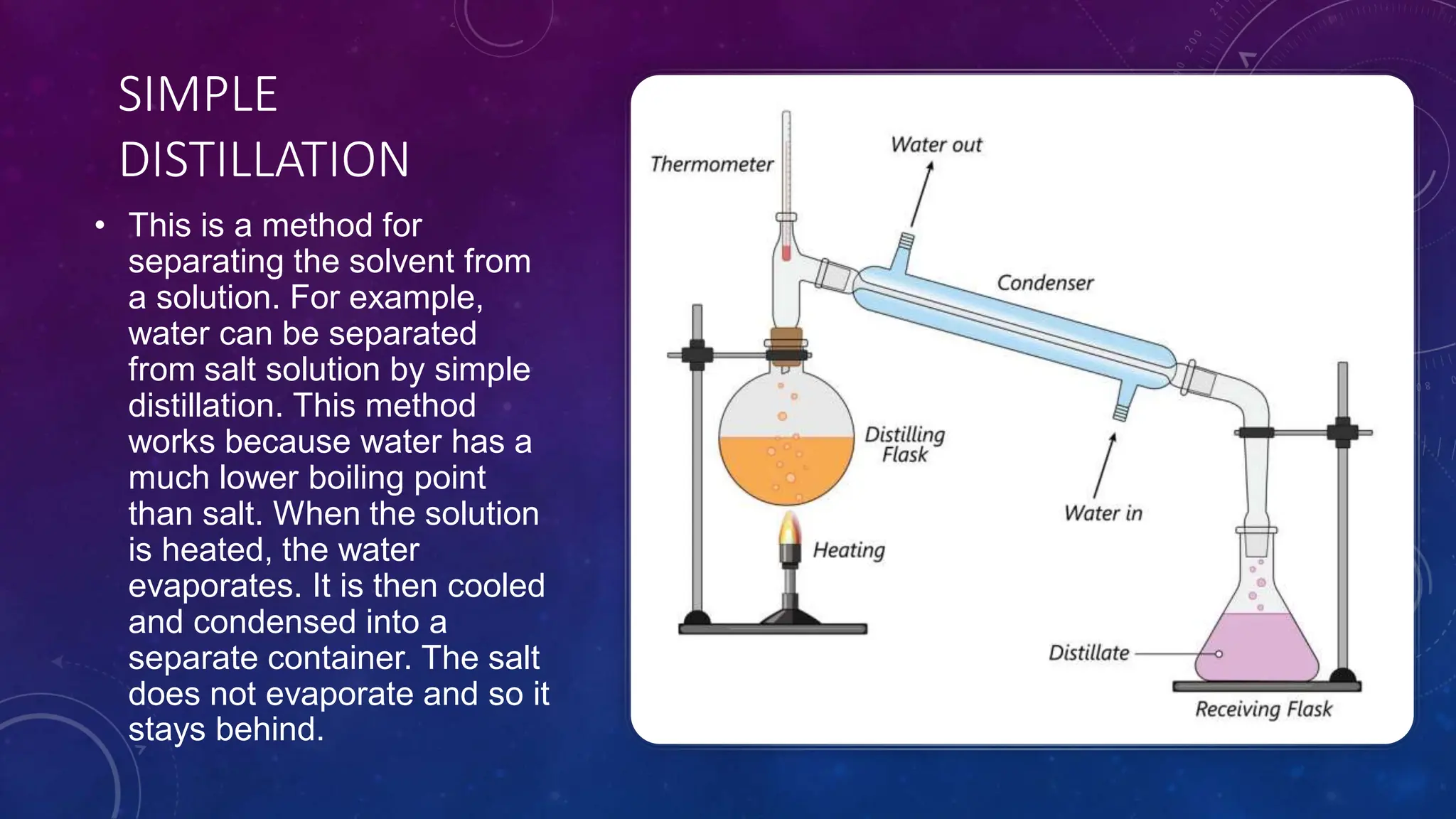
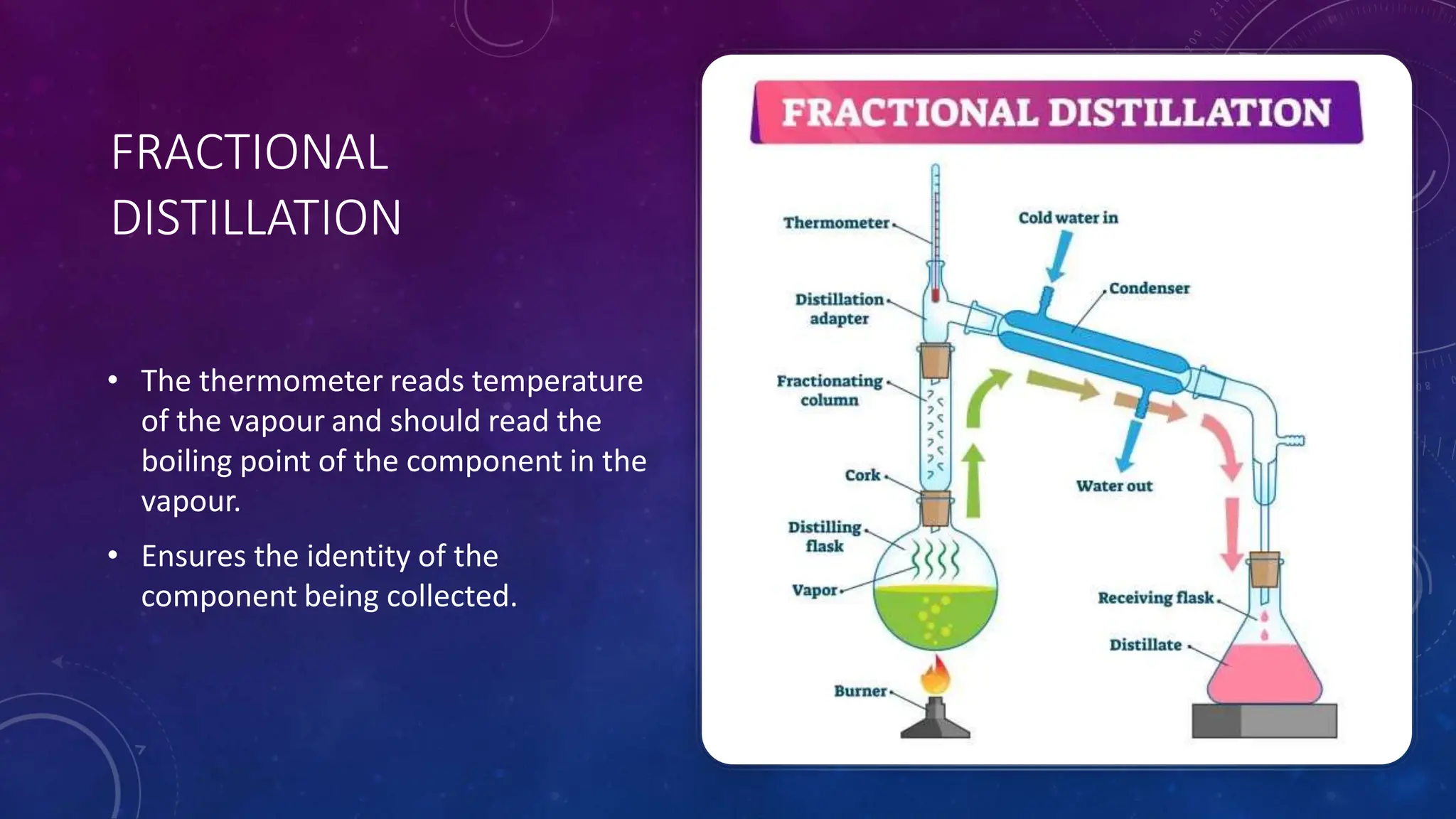
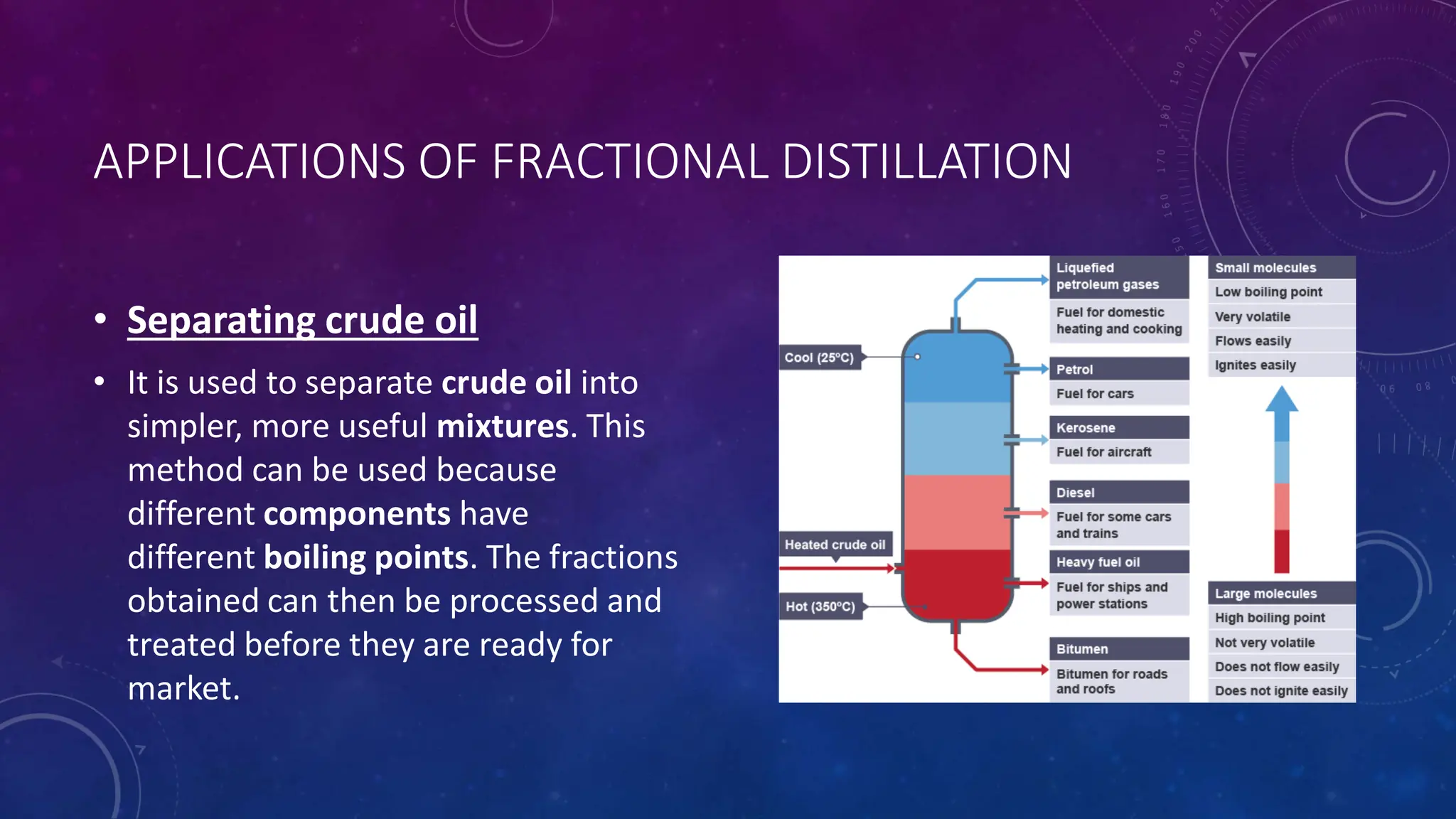
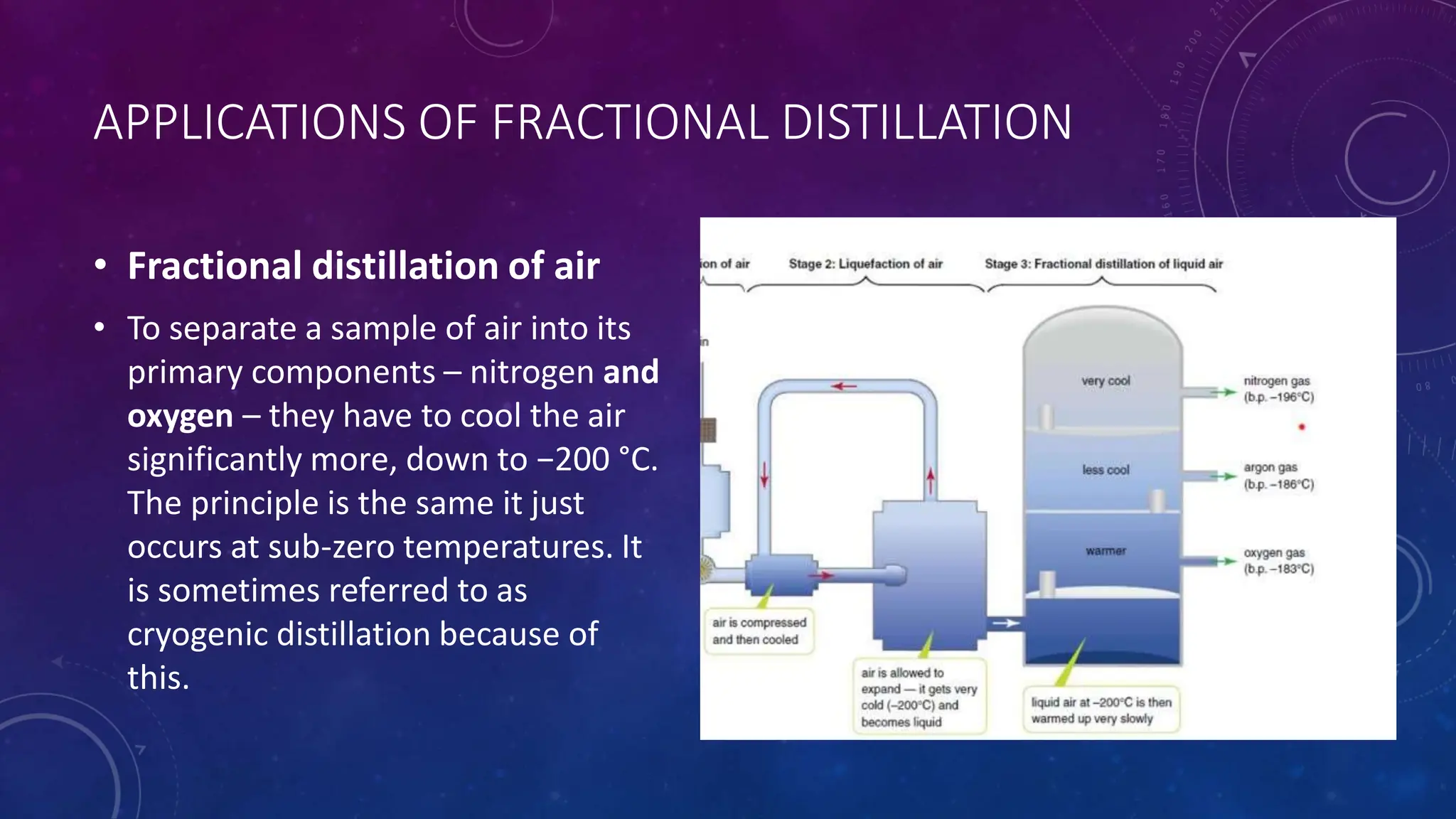
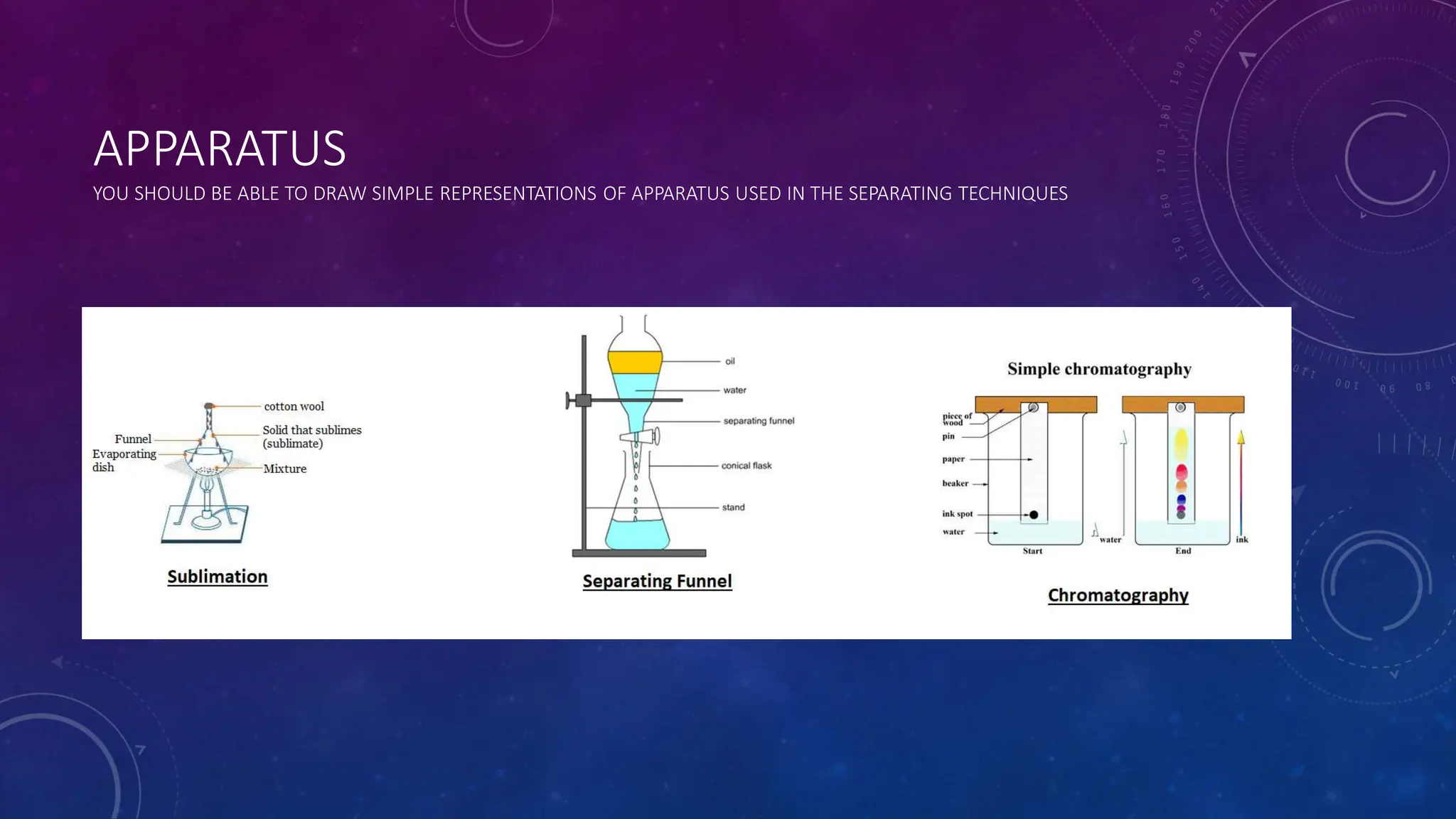
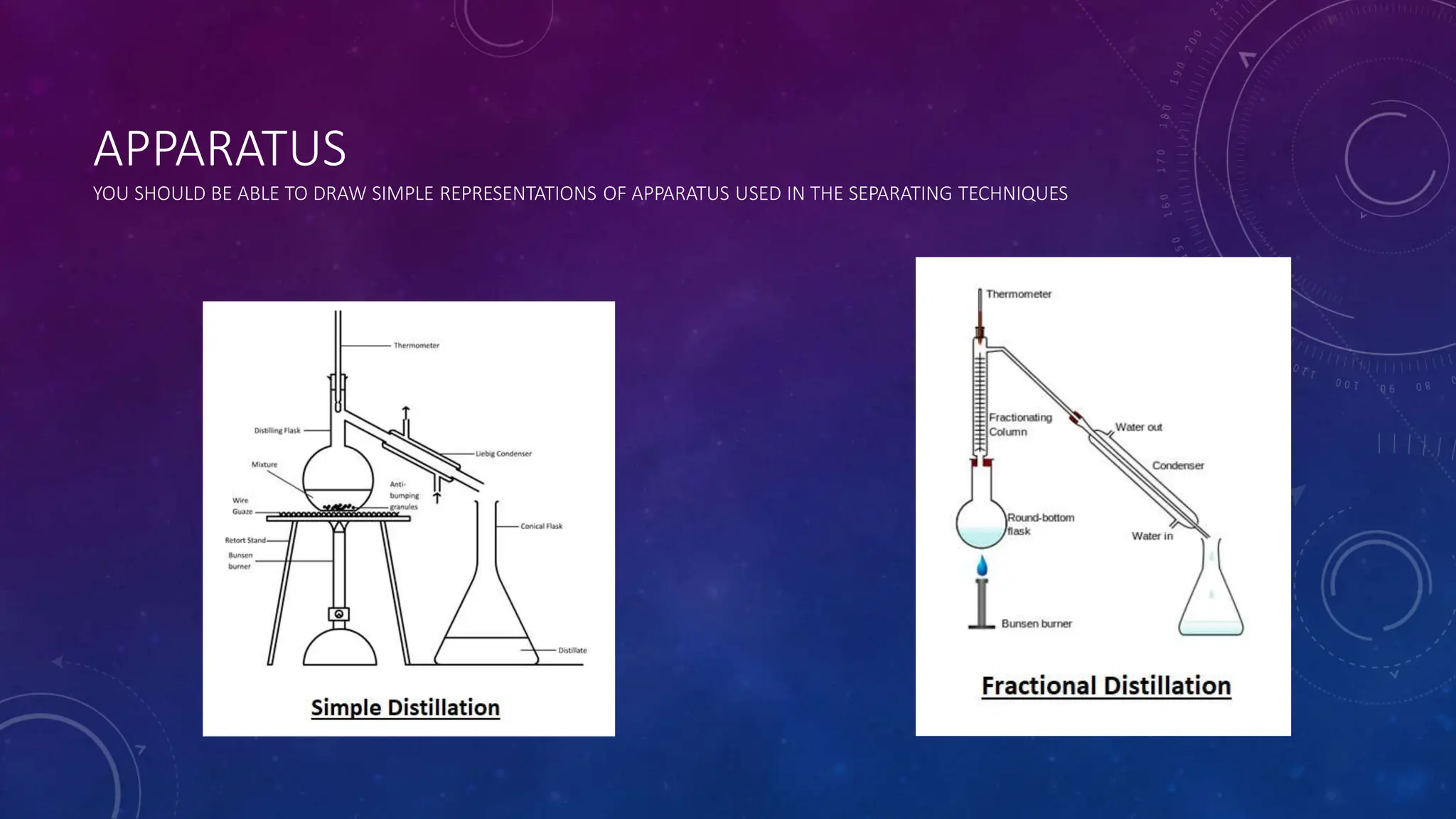
This document reviews various techniques for separating mixtures, emphasizing the importance of selecting methods based on the distinct properties of the components involved. Key techniques discussed include filtration, distillation (simple and fractional), crystallization, and chromatography, each suited for specific types of mixtures. Practical applications such as separating crude oil and producing ethanol through fermentation are also highlighted.